|
|
|
|
|
|
|
Previous: STRUCTURED ASSET SEC CORP COMM MORT PAS THR CERT SER 2000 C5, 8-K, EX-99.1, 2001-01-05 |
Next: DNA SCIENCES INC, S-1, EX-4.2, 2001-01-05 |
As filed with the Securities and Exchange Commission on January 5, 2001
Registration No. 333-
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER THE
SECURITIES ACT OF 1933
DNA SCIENCES, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 8731 | 77-0490090 | ||
| (State or Other Jurisdiction of Incorporation or Organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
DNA Sciences, Inc.
6540 Kaiser Drive
Fremont, CA 94555
(510) 494-4000
(Address, including zip code, and telephone number, including
area code, of registrant's principal executive offices)
Hugh Y. Rienhoff, Jr., M.D.
Chairman of the Board and Chief Executive Officer
DNA Sciences, Inc.
6540 Kaiser Drive
Fremont, CA 94555
(510) 494-4000
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Robert L. Jones, Esq. Laura A. Berezin, Esq. Cooley Godward LLP Five Palo Alto Square 3000 El Camino Real Palo Alto, CA 94306-2155 Phone: (650) 843-5000 Facsimile: (650) 849-7400 |
Thomas W. Christopher, Esq. Fried, Frank, Harris, Shriver & Jacobson One New York Plaza New York, NY 10004 Phone: (212) 859-8000 Facsimile: (212) 859-4000 |
Approximate date of commencement of proposed sale to the public:
As soon as possible after the effective date of this registration statement
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. / /
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. / /
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. / /
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. / /
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. / /
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered |
Proposed Maximum Aggregate Offering Price (1)(2) |
Registration Fee |
||
|---|---|---|---|---|
| Common Stock, $.001 par value | $125,000,000 | $31,250 | ||
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the commission, acting pursuant to said section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
Subject to Completion, dated January 5, 2001
PROSPECTUS
Shares
[LOGO]
Common Stock
This is our initial public offering of shares of common stock. We are offering shares. No public market currently exists for our common stock.
We have applied to have our common stock approved for quotation on the Nasdaq National Market under the symbol "DNAS." We anticipate the public offering price to be between $ and $ per share.
Investing in the shares involves risks. "Risk factors" begin on page 8.
| |
Per Share |
Total |
||||
|---|---|---|---|---|---|---|
| Public Offering Price | $ | $ | ||||
| Underwriting Discount | $ | $ | ||||
| Proceeds, before expenses, to DNA Sciences. | $ | $ | ||||
We have granted the underwriters a 30-day option to purchase up to shares of common stock on the same terms and conditions as set forth above to cover over-allotments, if any.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Lehman Brothers, on behalf of the underwriters, expects to deliver the shares on or about , 2001.
LEHMAN BROTHERS
CIBC WORLD MARKETS
DAIN RAUSCHER WESSELS
, 2001
—Text: "DNA Sciences' facility in Fremont, California"
—Text: "DNA Sciences' website"
—Text: "A DNA Sciences researcher"
| Prospectus Summary | 3 | |
| Risk Factors | 8 | |
| Forward-Looking Statements | 17 | |
| Use of Proceeds | 18 | |
| Dividend Policy | 18 | |
| Capitalization | 19 | |
| Dilution | 20 | |
| Selected Historical and Pro Forma Financial Data | 22 | |
| Management's Discussion and Analysis of Financial Condition and Results Of Operations |
24 | |
| Business | 31 | |
| Management | 52 | |
| Related Party Transactions | 64 | |
| Principal Stockholders | 67 | |
| Description of Capital Stock | 70 | |
| Shares Eligible for Future Sale | 73 | |
| Underwriting | 75 | |
| Legal Matters | 78 | |
| Experts | 78 | |
| Where You Can Find More Information | 78 | |
| Index to Financial Statements | F-1 |
Until , 2001 (25 days after the date of this prospectus), all dealers selling shares of our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the obligation of dealers to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus. This prospectus is not an offer to sell or a solicitation of an offer to buy our common stock in any jurisdiction where it is unlawful. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of common stock.
DNA SciencesSM, the DNA Sciences logoSM, The DNA Sciences Gene Trust ProjectSM, and The Gene Trust logoSM are servicemarks of DNA Sciences, Inc. Other trade names and trademarks appearing in this prospectus are the property of their respective holders.
The following summary is qualified in its entirety by the more detailed information and the financial statements and the notes to those financial statements appearing elsewhere in this prospectus.
We are a genetics discovery company focused on identifying the genetic basis of disease susceptibility, disease progression and response to drug treatment. We believe that the recent completion of the human genome sequence enables the discovery of previously unidentified variants in genes which contribute to disease. We refer to these variants as disease-associated gene variants. We believe that our innovative scientific approach will allow us to take advantage of the map of the human genome to find disease-associated gene variants both more quickly and with a higher rate of success than past efforts by others. Our scientific approach utilizes various study designs depending on what we know about a disease and its genetic basis. Our discovery efforts are enhanced by our team of accomplished geneticists, our strategy for recruiting patients and obtaining DNA samples and our state-of-the-art sequencing and genotyping facility. This facility has the capacity to sequence the equivalent of an entire human genome in fewer than 100 days. In addition, we have developed a prototype microchannel DNA sequencer that we believe will accelerate the ability to obtain human DNA sequence. We have licensed our technology and intellectual property which the DNA sequencer utilizes to Amersham Pharmacia Biotech in exchange for cash payments, research support, royalties, reagents at reduced cost and early access to the DNA sequencer.
We are focusing on diseases that have a significant genetic component, including cancer, asthma, inflammatory bowel disease, osteoporosis and diabetes. Many of these diseases are associated with gene variants that are difficult to find, most likely because they are only partially penetrant. This means that the gene variants do not always cause the disease in the person who inherits them. We believe that we are the only company focused on the discovery of partially penetrant gene variants. In addition to studying diseases with a known genetic component, we are examining diseases such as sudden infant death syndrome, or SIDS, that are suspected of having a genetic basis. Once we have identified disease-associated gene variants, we plan to develop diagnostic tests and, in the longer term, therapeutic products based on our discoveries.
In December 2000, we acquired PPGx, Inc., a company that provides genetic discovery services for pharmaceutical companies with a focus on gene variants that influence drug metabolism. This acquisition provides us with a government certified laboratory, results from genetic association studies in various diseases and access to more than 21,500 DNA samples on which these studies were based. Most of these samples were collected and analyzed by Sequana Therapeutics, Inc. and were contributed to PPGx by Axys Pharmaceuticals, Inc.
Our Approach
We use multiple methods to identify and validate disease-associated gene variants. These methods are intended to identify the region of the genome in which a disease-associated gene variant is present and to explore this region in detail. The method we select depends on a variety of factors, including the suspected penetrance of the gene variants we seek, the availability of DNA samples and the extent to which prior genetic studies have implicated specific genes or regions of the genome. We use genetic association studies involving large kinships of distantly related individuals to identify moderately penetrant genes. For more highly penetrant genes, we study multi-generational families with a recognizable pattern of inheritance. For diseases that share clinical similarities, such as sudden cardiac death in adults and SIDS, and where a specific genetic cause for one of the diseases has been established, we will explore these same genes in patients with the other disease. Once we have localized a region of DNA, we seek to identify all genes and their variants in that region. Using DNA sequencing, we plan to identify the specific disease-associated gene variants. We plan to validate our
3
findings by confirming the association between these identified gene variants and the disease in a large and diverse population of unrelated individuals.
We are using various means to recruit patients and obtain DNA samples. One approach is to identify kinships using the Utah Population Database and determine the prevalence of disease in those kinships using clinical histories. Another approach is to obtain DNA samples, candidate genes and data derived from family-based linkage analyses performed by third parties. We plan to use our Internet-based Gene Trust project to recruit patients for our validation studies and potentially to recruit affected families in order to identify disease-associated gene variants.
Our Commercial Focus
We intend to commercialize our discoveries of disease-associated gene variants by developing proprietary diagnostic tests and, where possible, therapeutic products. We expect our diagnostic tests will allow patients and healthcare providers to:
To date, we have initiated discovery and validation programs in multiple disease areas. Our most advanced effort is in SIDS, where we are currently sequencing five genes in 120 DNA samples to identify any gene variant that might be associated with the disease. In our cancer programs, we are currently identifying affected individuals and their kin through the Utah Population Database and the Utah Cancer Registry. The next step in these programs will be to recruit these individuals and begin an initial examination of their genomes. In our asthma, inflammatory bowel disease, osteoporosis and type II diabetes programs, we are currently undertaking a detailed examination of the genes and gene variants in the regions of the genome where a genetic link has been identified.
Our Strategy
Our goal is to become the world's leading genetics discovery company. To accomplish this goal, our strategy is to:
We were incorporated in May 1998 and changed our name to DNA Sciences, Inc. in May 2000. Our principal executive offices are located at 6540 Kaiser Drive, Fremont, California, and our telephone number is (510) 494-4000.
4
Unless otherwise indicated, information in this prospectus assumes that the underwriters do not exercise their over-allotment option and assumes the conversion of all of our preferred stock into common stock upon completion of the offering.
| Common stock we are offering | shares | |
Common stock to be outstanding immediately after this offering |
shares |
|
Use of proceeds |
To continue and expand our genetic discovery programs, maintain and expand our genetic database, commercialize diagnostic tests under development, purchase subscriptions to genomic databases from third parties, make capital expenditures, and for general corporate purposes, including working capital. See "Use of Proceeds." |
|
Proposed Nasdaq National Market symbol |
DNAS |
The number of shares of common stock to be outstanding after this offering is based on the number of shares outstanding as of December 29, 2000, and excludes:
5
The following table summarizes our financial data. You should read this information together with the financial statements and the notes to those statements appearing elsewhere in this prospectus and the information under "Selected Historical and Pro Forma Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations."
The statements of operations data displayed in the "Pro forma PPGx acquisition 1999" column for the year ended December 31, 1999 and in the "Pro forma PPGx acquisition 2000" column for the nine months ended September 30, 2000 are derived from the unaudited pro forma combined condensed financial statements included elsewhere in this prospectus. These pro forma data give effect to the PPGx acquisition as if it had taken place on January 1, 1999 and is based on our historical operating results and those of PPGx for the periods presented, giving effect to the amortization of tangible and intangible assets related to the acquisition. The pro forma information is not necessarily indicative of what actual financial results would have been had the acquisition taken place on January 1, 1999 and does not purport to indicate the results of future operations.
| |
|
|
|
|
|
|
Period from May 11, 1998 (inception) through September 30, |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Period from May 11, 1998 (inception) through December 31, 1998 |
Year Ended December 31, |
Nine Months Ended September 30, |
|||||||||||||||||||||
| |
|
Pro forma PPGx acquisition 1999 |
|
|
Pro forma PPGx acquisition 2000 |
|||||||||||||||||||
| |
1999 |
1999 |
2000 |
2000 |
||||||||||||||||||||
| |
(in thousands, except share and per share data) |
|||||||||||||||||||||||
| Statements of Operations Data: | ||||||||||||||||||||||||
| Revenues | $ | — | $ | 79 | $ | 966 | $ | — | $ | 500 | $ | 1,672 | $ | 579 | ||||||||||
| Operating expenses: | ||||||||||||||||||||||||
| Cost of revenues | — | — | 1,030 | — | — | 1,243 | — | |||||||||||||||||
| Research and development | 20 | 4,901 | 7,022 | 3,809 | 14,602 | 17,108 | 19,523 | |||||||||||||||||
| General and administrative | 309 | 2,700 | 6,518 | 1,995 | 4,652 | 8,109 | 7,661 | |||||||||||||||||
| Stock-based compensation | — | 418 | 530 | 129 | 5,985 | 6,069 | 6,403 | |||||||||||||||||
| Amortization of samples, goodwill and acquisition related intangibles | — | — | 9,158 | — | — | 6,869 | — | |||||||||||||||||
| Total operating expenses | 329 | 8,019 | 24,258 | 5,933 | 25,239 | 39,398 | 33,587 | |||||||||||||||||
| Loss from operations | (329 | ) | (7,940 | ) | (23,292 | ) | (5,933 | ) | (24,739 | ) | (37,726 | ) | (33,008 | ) | ||||||||||
| Interest income (expense), net | (19 | ) | 103 | 20 | 85 | 1,282 | 895 | 1,366 | ||||||||||||||||
| Net loss | $ | (348 | ) | $ | (7,837 | ) | $ | (23,272 | ) | $ | (5,848 | ) | $ | (23,457 | ) | $ | (36,831 | ) | $ | (31,642 | ) | |||
| Basic and diluted net loss per share | — | $ | (16.01 | ) | $ | (46.95 | ) | $ | (13.43 | ) | $ | (16.59 | ) | $ | (25.93 | ) | ||||||||
| Shares used in computing basic and diluted net loss per share | — | 489,382 | 495,686 | 435,342 | 1,413,990 | 1,420,294 | ||||||||||||||||||
| Pro forma basic and diluted net loss per share | $ | (1.49 | ) | $ | (1.56 | ) | ||||||||||||||||||
| Shares used in computing pro forma basic and diluted net loss per share | 5,243,896 | 15,011,622 | ||||||||||||||||||||||
6
The pro forma balance sheet data summarized below reflects:
The pro forma as adjusted balance sheet data summarized below includes the effect of the above adjustments and reflects:
| |
As of September 30, 2000 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
Actual |
Pro Forma |
Pro Forma As Adjusted |
||||||
(in thousands) |
|||||||||
| Balance Sheet Data: | |||||||||
| Cash and cash equivalents | $ | 40,882 | $ | 72,734 | |||||
| Total assets | 61,453 | 129,886 | |||||||
| Working capital | 31,148 | 60,910 | |||||||
| Total long-term liabilities | 5,520 | 5,520 | |||||||
| Redeemable convertible preferred stock | 69,809 | 135,320 | |||||||
| Deferred stock compensation | (15,205 | ) | (15,654 | ) | |||||
| Total stockholders' equity (net capital deficiency) | (24,263 | ) | (23,989 | ) | |||||
7
An investment in our common stock is risky. You should carefully consider the following risks as well as the other information contained in this prospectus. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Business
We have a limited operating history. We have not developed any products for commercial sale and we may not be able to do so.
Our company was founded in 1998, and we are still in an early stage of development. Accordingly, we have a limited operating history from which you can evaluate our business prospects. We cannot be certain that we will successfully develop and commercialize diagnostic and therapeutic products and services. Other than revenues derived from providing genotyping services, we have no revenues from the sale of products and services. We intend to develop diagnostic tests to help healthcare providers and patients assess susceptibility to particular diseases, the course of diseases and the appropriate treatment. However, we may be unable to successfully develop these products and services. Even if we are successful in developing these products and services, we do not expect our diagnostic tests to be available for commercial sale for many years. In addition, there may be no market for our diagnostic tests because of lack of patient or physician acceptance, competition or regulatory or reimbursement issues. For example, we may not be able to commercialize our diagnostic tests under development if those tests do not establish a significantly greater risk of disease susceptibility in order to justify their cost. If we cannot successfully develop or market our products, we may never become profitable.
We have a history of losses and negative cash flows, and anticipate continued losses for the foreseeable future.
We have incurred losses since our inception. On a pro forma basis, after giving effect to our acquisition of PPGx in December 2000, we had net losses of $36.8 million for the nine months ended September 30, 2000 and $23.3 million for the year ended December 31, 1999. As of September 30, 2000, we had an accumulated deficit of $31.6 million. We have not generated any revenue from the sale of products. We expect that in the near term any revenue will be derived from the sale of genotyping services, collaborations and grants. We expect to spend significant amounts over the next several years to discover disease-associated gene variants, further develop and commercialize our diagnostic tests, upgrade our production genotyping and informatics capabilities, improve and expand our facilities, complete the integration of PPGx and attract and retain skilled research, marketing and administrative personnel. In addition, in March 2000, we entered into an agreement with WebMD under which we are obligated to make payments to WebMD totaling $35.0 million over five years. As a result of our limited revenue and our expected expenses, we expect to incur operating losses and negative cash flows for the foreseeable future. We do not know when, if ever, we will achieve profitability or positive cash flows.
If our assumptions about our approach for discovering disease-associated gene variants are incorrect, we will be unable to successfully develop our diagnostic tests and other products and services.
Our approach for discovering and validating disease-associated gene variants is new and unproven, and to date, we have not discovered or validated any gene variants using it. This approach is based on scientific and statistical assumptions about how genetic variants are passed down from one generation to the next. Our approach also assumes that there is some association between genetic markers and gene variants responsible for a particular disease, and that we can discover this association. However, if the assumptions underlying our gene discovery methods prove incorrect, our approach may not be
8
successful in discovering disease-associated gene variants. If our approach fails, we will be unable to successfully develop diagnostic tests or therapeutics.
In addition to the assumptions upon which our approach is based, the fields of gene discovery, genomics and genetics are all based on the assumption that information about genes may help scientists better understand complex disease processes. Scientists generally have a limited understanding of the role of genes in diseases, and few products based on gene discoveries have been developed. We may discover that we are unable to determine the genetic basis for any particular disease. As a result, we cannot be certain that we will be able to develop any products based on discoveries of gene variants.
If we are not able to obtain sufficient additional funding to meet our expanding capital requirements, we may be forced to curtail or terminate our research programs and product development.
We have used and continue to use substantial amounts of cash to fund our activities. We expect our capital and operating expenditures to increase substantially over the next several years as we seek to discover disease-associated gene variants, develop and commercialize our diagnostic tests, upgrade our production technologies and facilities, complete the integration of PPGx and attract and retain skilled research, marketing and administrative personnel. Many factors will influence our future capital needs, including:
We believe that our current cash balances, together with the net proceeds of this offering and revenues to be derived from additional research services, collaborative research studies and genetic testing services, will be sufficient to fund our operations through 2002. To the extent our capital resources are insufficient to meet our future capital requirements, we will need to raise additional capital or incur new debt to fund our operations. We cannot be certain that additional equity or debt financing will be available on acceptable terms, or at all. If we are unable to obtain additional capital, we may be required to delay or reduce the scope of our genetic discovery programs or relinquish rights to technologies or products that we might otherwise seek to develop or commercialize.
The integration of PPGx will be expensive and may distract our management team from developing our products and services.
In December 2000, we acquired PPGx, and we are in the process of integrating this business into our existing operations. PPGx operates five facilities in different locations and employs 52 individuals. We expect that the integration of PPGx into our current operations will require us to make significant expenditures. We may encounter difficulties in assimilating the operations, personnel and services of PPGx with our own and operating a new business. For example, distance and cultural differences may make it difficult for us to successfully assimilate PPGx's operations located in five facilities in four states and England with our existing operations in Fremont, California. In addition, this acquisition may expose us to various unknown liabilities and may result in the loss of key employees of PPGx. In
9
addition, this integration effort may divert our management's attention from the development of our products and services.
Ethical, legal and social issues related to the use of genetic information and genetic testing may cause less demand for our products.
The prospect of more extensive genetic testing has focused public attention on the appropriate uses of the resulting information. For example, individuals might use genetic testing to decide whether or not to take out life or health insurance on themselves or their families, making insurance more costly for those who elect coverage. Likewise, concerns have been expressed that insurance carriers might use such tests to establish rates for individuals or groups, raising premiums to prohibitive levels. In either case, the prospect of health or life insurance rate setting or policy issuance based on genetic profiling could result in barriers to the acceptance of such tests by consumers. If so, demand for our diagnostic products could be significantly reduced. Similarly, use of genetic profiling by employers or by governmental authorities could create a backlash that might result in new laws that limit the use of information derived from genetic testing. Any of these scenarios could reduce or delay the development of the potential markets for our products.
Concerns about privacy of genetic information provided to us or about our informed consent procedures may limit participation in our Gene Trust project, which could cause a delay in the completion of our genetic studies or subject us to liabilities.
The success of our business depends, in part, on our ability to acquire patient populations for our genetic studies. One of our sources of patient populations is our Gene Trust project. The success of this project depends on the participation of volunteers through our DNA.com website. We may be unable to attract a sufficient number of participants to the project because of concerns regarding the privacy of genetic and personal information provided to us over the Internet. If we are unable to attract a sufficient number of participants, our studies could be delayed while we seek additional patients to validate our results.
Furthermore, some third parties have publicly raised concerns regarding the privacy and informed consent procedures we follow in recruiting volunteers for studies over the Internet. While our study protocol has received institutional review board approval, we cannot be certain that third parties will not continue to raise concerns about our programs. These concerns may harm our reputation or affect the willingness of visitors to DNA.com to participate in our Gene Trust project.
In addition, numerous state and federal laws govern the collection, dissemination, use, access to and confidentiality of patient health information. All states have laws and regulations that protect the confidentiality of medical records or medical information, and many states have laws that specifically regulate genetic testing and the privacy of genetic information. In addition, the federal Department of Health and Human Services has recently published final regulations implementing the Health Insurance Portability and Accountability Act of 1996, or HIPAA, concerning the confidentiality of individually identifiable health information that will affect our use of samples collected by healthcare providers. The application of these laws to the information we collect and disseminate could create potential liability under these laws. We have designed our informed consent process to comply with applicable state and federal laws, and have designed our network and data management procedures to protect the identity of participants and maintain the security of our data. However, in the event that confidential information about any participant is inadvertently disclosed, we may be liable for damages that occur as a result of this disclosure and may be subject to regulatory actions under state or federal laws.
10
Regulation of the Internet could impact the way in which we collect DNA samples from participants in the Gene Trust project.
Internet user privacy has become an issue both in the United States and abroad. Whether and how existing privacy and consumer protection laws in various jurisdictions apply to the Internet is uncertain and may take years to resolve. Any legislation or regulation of this nature could affect the way we conduct our business, particularly in our collection of DNA samples through the Gene Trust project. Furthermore, activities involving the use of the Internet in the healthcare arena have come under increased investigation by the Federal Trade Commission, or FTC, and state consumer protection agencies. In fact, many Internet-based healthcare companies have received requests for information from the FTC concerning website privacy policies and practices. Although we have not ourselves received any inquiry from the FTC or other governmental entities, future government inquiries could divert our attention from our business matters, create unfavorable publicity and harm our reputation.
We depend on third parties, such as the University of Utah, to obtain access to patients for our genetic studies. If we fail to maintain these relationships and develop new relationships, we may be unable to complete our genetic studies and develop our diagnostic tests.
We have entered into a research agreement with the University of Utah to collaborate with University researchers to determine the gene variants which contribute to diseases we are studying. Working with us, these researchers will access the Utah Population Database to identify kinships with disproportionate occurrences of disease. The Utah Population Database is critical to our approach to discovering gene variants that contribute to disease and represents the largest source of patients for our studies. We believe that there are very few other databases in the world that are as useful to genetic studies. Our access to the database or any collaborative efforts with University researchers can be terminated by the University of Utah upon 30 days notice. If we fail to maintain our relationship with the University of Utah for access to this database, we may be unable to gain access to a similar database that identifies similar kinships. If we are unable to access a comparable database, our genetic studies and development of our diagnostic tests would be delayed.
Furthermore, if we fail to maintain our relationships with our sponsored researchers at the University of Utah Medical Center, it will be necessary to retain other sponsored researchers to conduct our studies at the University, including the use of the database. Identifying and retaining new consultants capable of using the database would be time consuming and may be expensive. As a result, we may experience delays in our studies.
We are establishing collaborations with other academic institutions and investigators to recruit patients of specific ethnicities or with particular diseases or special clinical information. If we fail to establish these collaborations, our product development efforts could be delayed.
We may be required to obtain licenses from or make payments to third parties in order to use some of the DNA samples acquired from PPGx which could limit the revenues we generate from these samples or harm our ability to commercialize these products.
Some of the DNA samples we acquired from PPGx or data derived from these samples may be subject to contractual rights of third parties under the terms of the original transfer agreements to Sequana Therapeutics. These rights may require us to obtain licenses from or make payments to these third parties to commercialize products or services based on the use of these samples. Also, our rights to use some of the DNA samples from asthma patients and derivative data derived from these samples may be terminated. If any of these events were to occur, our revenues could be limited or our ability to commercialize products and services based on these samples could be harmed.
11
We may not be able to compete successfully with biotechnology companies and established pharmaceutical companies in the development and marketing of products based on gene variants contributing to disease.
A number of companies are attempting to rapidly identify and patent gene variants that cause disease or an increased susceptibility to disease. Competition in this field is intense and is expected to increase. We have numerous competitors, including major pharmaceutical and diagnostic companies, specialized biotechnology firms, universities and other research institutions and the Human Genome Project and other government-sponsored entities. Our competitors may develop new technologies to allow them to identify genes and gene variants that contribute to disease before we do. We also face competition in the development of our diagnostic products from larger and more established diagnostic companies. In addition, genotyping technology companies may enter the gene discovery business. Competition is also intense in the genetic database area. Companies may in the future direct their efforts to developing databases containing clinical and genetic information that would compete with our own database. In addition, PPGx faces competition in providing genotyping services from universities and laboratory service and biotechnology companies. If other companies are able to discover gene variants that contribute to disease, develop diagnostic products or services or develop proprietary databases before we do, our business may not succeed.
Many of our competitors have considerably greater capital resources, research and development staffs and facilities and technical and other resources than we do. In addition, the barriers to entry in the area of gene discovery are relatively low, and the patent protection available for our products and discoveries is unclear. We believe our approach to the discovery of disease-associated gene variants may be replicated by competitors. Moreover, because access to the Utah Population Database is not licensed to us on an exclusive basis, competitors may obtain access to this database to conduct studies that cover the same diseases that we are studying. We may also face competition from other entities in gaining access to other sources of DNA samples used for research and development purposes.
Competitors have established, and in the future may establish, patent positions with respect to gene sequences or variants that are included in our genetic studies. These patent positions or the public availability of gene sequences comprising substantial portions of the human genome could decrease the commercial value of the results of our studies, making it more difficult for us to compete. In addition, if any of our competitors discover gene variants that contribute to disease, they may obtain patent protection for those gene variants or any derivative products.
We may not be able to protect the proprietary rights that are critical to our success.
We have filed U.S. patents covering technology for identifying gene variants, methods of genetic analysis using gene variants and business methods relating to the acquisition and transfer of information regarding gene variants over the Internet. We intend to protect our proprietary position by filing United States and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. Specifically, we intend to file patent applications relating to gene variants we discover and to newly identified associations between gene variants and traits.
Our commercial success will depend in part on obtaining patent protection for gene variants, associations between gene variants and diseases and related subject matter, such as diagnostic tests. Patent law relating to the scope of claims in the area of genetics and gene discovery is generally uncertain and still evolving and involves complex legal and factual considerations. There is substantial uncertainty regarding the patentability of genes or gene fragments without known functions. The U.S. Patent and Trademark Office initially rejected a patent application by the National Institutes of Health on partial genes. There is also uncertainty regarding the patentability of Internet-based business methods. Accordingly, the degree of future protection is uncertain, and we cannot predict the breadth of claims allowed in any patents issued to us or others. We cannot be sure that any of our pending or
12
future patent applications will result in issued patents or that we will develop additional proprietary technologies that are patentable. In addition, any patents issued to us may not serve as a basis for commercially viable products or provide us with any competitive advantages or will not be challenged by third parties.
We are aware that third parties are engaged in sequencing of human DNA, discovery of gene variants, characterization of gene function and determining associations between gene variants and disease. Numerous patent applications have been filed and will in the future be filed by other entities directed to genes, partial gene sequences, gene variants, their uses and related subject matter. Many of these have issued as patents in the United States and other countries. We expect that some of the genes or gene variants that we use in our genetic studies and the development of our products are or will be subject to patent coverage of others. In addition, we are aware of several issued patents by third parties directed to technology for detection of gene variants. Any claim of patent infringement that is successfully asserted against us could subject us to liability for damages or result in an injunction prohibiting the sale of our products and services. We could also incur substantial costs in litigation if we are required to defend ourselves in patent suits brought by third parties or if we initiate suits to assert or protect our intellectual property rights.
In addition, third parties may develop products that are similar or identical to our products. We cannot be certain that our patent applications will have priority over patent applications of others. Even if we are granted patents, we cannot be sure that they would be valid and enforceable against third parties. If human DNA sequence, gene variants, gene functions and associations are the subject of patent filings by others, or become publicly available before we apply for patent protection, our ability to obtain patent protection for this subject matter could be adversely affected. Further, a patent does not provide the patent holder with freedom to operate in a way that infringes the patent rights of others. Defense of patent infringement claims, even if successful, would likely be expensive and time consuming. In addition, we may need to obtain a license in order to continue to market our products and services or, alternatively, modify our products and services to avoid patent coverage. We cannot be certain that any license required under any patent would be made available on commercially acceptable terms, if at all. If licenses are not available, we may be required to cease conducting our studies or marketing our products or services.
While we require employees, academic collaborators and consultants to enter into confidentiality agreements where appropriate, others may gain access to our proprietary information. If this occurs, our competitive position would suffer.
If we are unable to obtain regulatory approvals for diagnostic or therapeutic products resulting from our gene discovery programs, we will not be able to derive revenues from these products.
The manufacture and sale of medical diagnostic devices intended for commercial use are subject to extensive government regulation in the United States and other countries. The process of obtaining FDA and other required regulatory approvals can be time-consuming, expensive and uncertain, frequently requiring several years from the commencement of clinical trials to the receipt of regulatory approval. We may not be able to obtain necessary regulatory approvals or clearances or comply with regulatory requirements applicable to in vitro diagnostic tests in the United States or internationally on a timely basis, or at all. Noncompliance with applicable requirements can result in failure of the government to grant premarket clearance or approval for devices, withdrawal of clearances or approvals, total or partial suspension of production, fines, injunctions, civil penalties, recall or seizure of products and criminal prosecution. We intend to commercialize our diagnostic products and services either by selling diagnostic test kits to physicians, laboratories and hospitals or by performing diagnostic test services ourselves. Development and performance of diagnostic test services within a clinical laboratory is currently not subject to FDA regulation. However, it is possible that the FDA will choose to regulate such testing services in the future. Additionally, if we choose to sell these kits to physicians,
13
hospitals and laboratories, they would be considered medical devices for which we would be required to obtain FDA approval. The regulatory process can take many years and require substantial resources. We cannot predict whether this regulatory approval would be obtained. Furthermore, regulatory approval may impose limitations on the use of our diagnostic products. After initial regulatory approval, a marketed product and its manufacturer are subject to continuing review. We could be required to withdraw any diagnostic product from the market if we discover previously unknown problems. The FDA's regulatory requirements for drugs and biological products is as extensive or more extensive than that for medical devices requiring pre-market approval. The regulatory process can take many years and require substantial resources. We may not be able to obtain all required regulatory approvals.
In connection with performing clinical testing services, we operate a facility registered under the Clinical Laboratory Improvement Act of 1967 and the Clinical Laboratory Improvement Amendments of 1988. These laws are collectively referred to as CLIA. CLIA is intended to ensure the quality and reliability of clinical laboratories in the United States. Many states also impose laboratory licensure requirements. While our facility is currently certified under CLIA, we may not be able to maintain our CLIA certification or able to obtain and maintain all necessary state laboratory authorizations. If we lose our certification, or fail to obtain or maintain all necessary state laboratory authorizations, we would be unable to perform these services.
Currently, as a matter of its enforcement discretion, FDA does not regulate diagnostic tests developed in CLIA laboratories certified to conduct high complexity testing. Although FDA views such tests to be medical devices subject to the agency's authority, FDA has chosen through an exercise of its enforcement discretion to not require laboratories developing such tests, referred to as "home brew" or "in-house developed" tests, to seek approval or clearance of those tests at the present time. FDA has developed its home brew policy to permit laboratories meeting certain, specific operating criteria to continue to operate. FDA may change these criteria or end its home brew policy. If FDA chooses to regulate such tests, we could be required to obtain FDA approval to market our home brew tests. We cannot be certain that this regulatory approval would be obtained.
Our inability to obtain adequate reimbursement for our products could seriously harm our business, financial condition and results of operations.
Our ability to successfully commercialize diagnostic and therapeutic products developed by us may depend on the extent to which such products are reimbursed by third-party payers. Increasingly, third-party payers are limiting reimbursement for healthcare products and services. There can be no assurance that any third-party insurance coverage will be available for any products developed by us. If adequate coverage and reimbursement levels are not provided for our products, the market acceptance of these products may be reduced, which may have a material adverse effect on our business, financial condition and results of operations.
If we fail to maintain or obtain rights to third-party technology, our ability to discover disease-associated gene variants or to develop commercial products based on those discoveries could be delayed.
As we seek to identify disease-associated gene variants, we expect to subscribe to genomics databases from third parties. If we fail to obtain access to these databases, the progress of our gene discovery programs may be delayed. Additionally, our success is dependent on our ability to enter into licensing arrangements with commercial or academic entities for technology that is advantageous or necessary to the development and commercialization of our technologies. We may not be able to negotiate additional license agreements in the future on acceptable terms, or at all.
We may be unable to hire and retain the key personnel upon whom our success depends.
We depend on Hugh Y. Rienhoff, Jr., Founder, Chairman and Chief Executive Officer, and Ray White, Chief Scientific Officer. If either of these individuals leaves DNA Sciences, our genetic discovery programs will likely be delayed. Our future success also depends on our ability to attract, hire and retain additional personnel, particularly experienced geneticists. There is intense competition for qualified personnel and the turnover rate for personnel in our industry is high. As a result, we cannot be certain that we will be able to continue to attract and retain needed personnel. If we fail to attract and retain key personnel, our ability to compete could be jeopardized.
14
Use of any diagnostic products developed by us may result in liability claims.
Patients who have received a diagnostic test developed from our gene discovery programs may bring product liability claims against us if the results of our test are found to be inaccurate. For example, a claim could be based on allegations that one of our tests failed to identify, in a particular patient, a gene variant associated with a particular disease and as a consequence, the patient failed to take appropriate action to prevent or manage the condition. We currently do not carry liability insurance to cover these claims. We are not certain that we will be able to obtain this insurance or, if obtained, that sufficient coverage can be acquired at a reasonable cost. If we cannot protect against these potential liability claims, we may find it difficult or impossible to commercialize our products. A liability claim or product recall could have a material adverse effect on our financial condition.
Our operations involve a risk of injury and contamination from hazardous materials, which could be very expensive to us.
Our activities involve the generation, use and disposal of hazardous materials and wastes, including various chemicals. The risk of accidental contamination or injury from these materials cannot be completely eliminated. If an accident involving these substances occurs, we could be liable for any damages that result, which could seriously harm our business. We are subject to laws and regulations governing the use, storage, handling and disposal of these materials, including standards prescribed by the U.S. Environmental Protection Agency. If we were found not to be in compliance with these regulations or if additional regulations were issued, we may be required to incur significant compliance costs, which could have a material adverse effect on our operations.
We are in the process of moving our genotyping facilities and principal executive offices to a new facility, which could cause delays in the development and commercialization of our products.
We are currently moving most of our operations to a new facility in Fremont, California. Our schedule calls for this move to be completed in the first quarter of 2001. If the process of moving our genotyping equipment and setting up our operations in this new facility is delayed, the development and commercialization of our technologies may be interrupted or delayed.
Risks Related to the Offering
The interests of our controlling stockholders may conflict with our interests and the interests of our other stockholders.
Immediately after this offering, our executive officers, directors and principal stockholders, and their respective affiliates, will beneficially own approximately % of our outstanding common stock. These stockholders, if acting together, would be able to control substantially all matters requiring approval by our stockholders, including the election of all directors and approval of significant corporate transactions. This could have the effect of delaying or preventing a change of control and will make some transactions difficult or impossible without the support of these stockholders. See "Principal Stockholders" for details on our stock ownership.
Our right to issue preferred stock and anti-takeover provisions could make a third-party acquisition of us difficult and otherwise adversely affect common stockholders.
Upon the closing of this offering, our board of directors will be authorized to designate and issue up to 5,000,000 shares of preferred stock and determine the price, rights, preferences and privileges of those shares without any further vote or action by our stockholders. If you own common stock, your ownership rights will be subject to, and may be adversely affected by, the rights of the owners of preferred stock that we may issue in the future. As a result, the issuance of preferred stock could have a material adverse effect on the market value of the common stock. In addition, issuances of preferred
15
stock could make it more difficult for a third party to acquire a majority of our outstanding voting stock.
Upon the closing of this offering, our certificate of incorporation will provide that our board of directors will be divided into three classes, each serving staggered three-year terms. Accordingly, stockholders may elect only a minority of our board at any annual meeting, which may have the effect of delaying or preventing a change in management or control of DNA Sciences.
Further, we are subject to the anti-takeover provisions of Section 203 of the Delaware General Corporation Law. Under this law, if anyone becomes an "interested stockholder" in DNA Sciences, we may not enter into a "business combination" with that person for three years without special approval. These provisions could delay or prevent a change of control. Certain other provisions of our certificate of incorporation and bylaws could also delay or prevent changes of control or management. These provisions could adversely affect the market price of the common stock.
Our common stock has never been publicly traded and we cannot predict the extent to which a trading market will develop.
Before this offering, there has been no public market for our common stock. We cannot predict the extent to which an active public market for the common stock will develop or be sustained after this offering. We will negotiate the initial public offering price with the representatives of the underwriters. The initial public offering price of our common stock may not be indicative of future market prices.
Market prices of emerging biotechnology companies have been highly volatile, and the market for our stock may exhibit volatility as well.
The market price of our common stock is likely to be highly volatile. In addition to various risks described elsewhere in this prospectus, the following factors could also cause price volatility:
Extreme price and volume fluctuations occur in the stock market from time to time and can particularly affect the prices of technology and biotechnology stocks. These extreme fluctuations are often unrelated to the actual performance of the affected issuers. These broad market fluctuations may adversely affect the market price of our common stock.
Future sales by our current stockholders may adversely affect our stock price and our ability to raise funds in new stock offerings.
Sales of our common stock by our current stockholders in the public market after this offering could cause the market price of our stock to fall. Sales may also make it more difficult for us to sell equity securities or equity-related securities in the future at a time and price that our management deems acceptable, or at all. Upon the completion of this offering, we will have shares of common stock outstanding, assuming no exercise of options or warrants after December 29, 2000 and
16
assuming no exercise of the underwriters' over-allotment option. Of these outstanding shares of common stock, the shares sold in this offering will be freely tradable, without restriction under the Securities Act of 1933, unless purchased by our "affiliates." The remaining 26,193,788 shares of common stock held by existing stockholders are "restricted securities" and may be resold in the United States public market only if registered or pursuant to an exemption from registration.
Immediately following the completion of this offering, holders of 21,460,689 shares of common stock and warrants to purchase an aggregate of 254,000 shares of common stock will be entitled to certain registration rights. Upon registration, these shares may be freely sold in the public market.
All of our current stockholders have agreed that they will not sell any shares of common stock for a period of 180 days after the date of this prospectus without the prior written consent of the underwriters.
Upon expiration of the lock-up agreements:
Purchasers in this offering will suffer immediate dilution.
If you purchase common stock in this offering, you will experience dilution, which means that the value of your shares based upon our actual book value will immediately be less than the offering price you paid. Based upon the pro forma net tangible book value of the common stock at September 30, 2000, your shares will be worth $ less per share than the price you paid in the offering. If options and warrants we previously granted are exercised, additional dilution is likely to occur. Following the completion of this offering, options to purchase 4,573,364 shares of common stock at a weighted average exercise price of $1.58 per share and warrants to purchase 254,000 shares of common stock at a weighted average price of $7.50 per share will be outstanding.
This prospectus contains forward-looking statements. These statements relate to future events or our future financial performance. In some cases, you can identify forward-looking statements by terminology such as "may," "will," "should," "could," "expect," "plan," "anticipate," "believe," "estimate," "predict," "intend," "potential" or "continue," the negative of such terms or other comparable terminology. These statements are not guarantees of future performance and are subject to risks, uncertainties and other factors, some of which are beyond our control, are difficult to predict and could cause actual results to differ materially from those expressed, implied or forecasted in the forward-looking statements. In addition, the forward-looking events discussed in this prospectus might not occur. These risks and uncertainties include, among others, those described in "Risk Factors" and elsewhere in this prospectus. Readers are cautioned not to place undue reliance on these forward-looking statements, which reflect our management's view only as of the date of this prospectus. Except as required by law, we undertake no obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.
17
We estimate that we will receive net proceeds from this offering of about $ million, or about $ million if the underwriters exercise their over-allotment option in full, at an assumed initial public offering price of $ per share, after deducting the underwriting discount and estimated offering expenses.
We expect to use approximately $ million to continue and expand our genetic discovery programs, maintain and expand our genetic database, commercialize diagnostic tests under development and to purchase subscriptions to genomic databases from third parties.
We also intend to spend approximately $ million of the net proceeds of this offering for capital expenditures. These expenditures will include the development or purchase of:
The remainder of the net proceeds are expected to be used for working capital and general corporate purposes. We may also use a portion of the net proceeds to acquire businesses, technologies, or products complementary to our business; however, we do not currently have any specific plans to do so.
Pending use of the net proceeds for the purposes described above, we intend to invest the net proceeds in short-term, interest-bearing, investment-grade securities.
We have never declared or paid any dividends on our capital stock. We currently expect to retain future earnings, if any, for use in the operation and expansion of our business and do not anticipate paying any cash dividends in the foreseeable future.
18
The following table sets forth our capitalization as of September 30, 2000:
This table should be read with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our financial statements and notes appearing elsewhere in this prospectus.
| |
As of September 30, 2000 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Actual |
Pro Forma |
Pro Forma As Adjusted |
|||||||||
| |
(in thousands) |
|||||||||||
| Long-term portion of equipment financing obligations | $ | 5,520 | $ | 5,520 | $ | 5,520 | ||||||
| Redeemable convertible preferred stock, $0.001 par value; 22,000,000 shares authorized, actual and pro forma; 5,000,000 shares authorized, pro forma as adjusted; 15,382,006 and 21,461,014 shares issued and outstanding, actual and pro forma, respectively; no shares outstanding pro forma as adjusted | 69,809 | 135,320 | — | |||||||||
| Stockholders' equity (net capital deficiency): | ||||||||||||
| Common Stock, $0.001 par value; 40,000,000 shares authorized, actual and pro forma; 75,000,000 shares authorized, pro forma as adjusted; 4,075,708 shares issued and outstanding, actual; 4,082,012 shares issued and outstanding, pro forma; shares issued and outstanding, pro forma, as adjusted | 4 | 4 | ||||||||||
| Additional paid-in capital | 22,824 | 25,247 | ||||||||||
| Notes receivable from stockholders | (244 | ) | (244 | ) | (244 | ) | ||||||
| Deferred stock compensation | (15,205 | ) | (15,654 | ) | (15,654 | ) | ||||||
| Deficit accumulated during the development stage | (31,642 | ) | (33,342 | ) | (33,342 | ) | ||||||
| Total stockholders' equity (net capital deficiency) | (24,263 | ) | (23,989 | ) | ||||||||
| Total capitalization | $ | 51,066 | $ | 116,851 | $ | |||||||
This table excludes the following shares as of December 29, 2000:
19
If you invest in our common stock, your interest will be diluted to the extent of the difference between the public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of common stock after this offering. Pro forma net tangible book value per share represents the amount of our pro forma total tangible assets less total liabilities, divided by the pro forma number of shares of common stock outstanding. As of September 30, 2000, our pro forma net tangible book value was approximately $93.2 million or $3.65 per share of common stock after giving effect to:
Without taking into account any other changes in our net tangible book value after September 30, 2000, other than to give effect to this offering of shares of common stock at an assumed initial offering price of $ per share, less the underwriting discount and estimated offering expenses, our pro forma as adjusted net tangible book value, at September 30, 2000, would have been approximately $ or $ per share. This represents an immediate increase in the pro forma net tangible book value of $ per share of our common stock to current stockholders and an immediate dilution of $ per share to new investors. The following table illustrates this dilution on a per share basis:
| Assumed initial public offering price per share | $ | ||||||
| Pro forma net tangible book value per share at September 30, 2000 | $ | 3.65 | |||||
| Increase per share attributable to new investors | |||||||
| Pro forma as adjusted net tangible book value per share after this offering | |||||||
| Dilution per share to new investors | $ | ||||||
The following table summarizes, on a pro forma basis as of September 30, 2000 after giving effect to the sale of Series C preferred stock and Series C-1 preferred stock, and the issuance of Series D preferred stock and common stock in connection with the acquisition of PPGx in December 2000, the differences between the number of shares purchased from us, the total consideration paid and the average price per share paid by the existing and the new investors. We have assumed an initial public offering price of $ per share, no exercise of the underwriters' over-allotment option and we have not deducted the underwriting discount and estimated offering expenses in our calculations.
| |
Shares Purchased |
Total Consideration |
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Average Price Per Share |
||||||||||||
| |
Number |
Percent |
Amount |
Percent |
|||||||||
| Existing shareholders | 25,543,426 | $ | 138,496,000 | $ | 5.42 | ||||||||
| New investors | |||||||||||||
| Total | 100.0 | % | $ | 100.0 | % | $ | |||||||
The number of shares of common stock outstanding in the table above is based on the number of shares outstanding as of December 29, 2000, and excludes:
20
The exercise of outstanding options and warrants having an exercise price less than the initial public offering price would increase the dilutive effect to new investors.
21
SELECTED HISTORICAL AND PRO FORMA FINANCIAL DATA
The following selected financial data should be read in conjunction with the financial statements and the notes to those statements included elsewhere in this prospectus, and "Management's Discussion and Analysis of Financial Condition and Results of Operations." The statements of operations data for the period from May 11, 1998 (inception) to December 31, 1998 and the fiscal year ended December 31, 1999 and the balance sheet data at December 31, 1998 and 1999 are derived from our financial statements included elsewhere in this prospectus that have been audited by Ernst & Young LLP, independent auditors. The statements of operations data for the nine-month periods ended September 30, 1999 and September 30, 2000 and the balance sheet data as of September 30, 2000 are derived from unaudited financial statements included elsewhere in this prospectus. Historical results are not necessarily indicative of future results.
The statements of operations data displayed in the "Pro Forma PPGx Acquisition 1999" column for the year ended December 31, 1999 and in the "Pro Forma PPGx Acquisition 2000" column for the nine months ended September 30, 2000 are derived from the unaudited pro forma combined condensed financial statements included elsewhere in this prospectus. These pro forma data give effect to the PPGx acquisition as if it had taken place as of January 1, 1999 and is based on our historical operating results and those of PPGx for the periods presented, giving effect to the amortization of intangible assets related to the acquisition. These amortization amounts have also been derived from our unaudited combined condensed financial statements included elsewhere in this prospectus. The pro forma information is not necessarily indicative of what actual financial results would have been had the acquisition taken place on January 1, 1999 and does not purport to indicate the results of future operations.
| |
|
Year Ended December 31, |
|
|
|
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Period from May 11, 1998 (inception) through December 31, 1998 |
Nine Months Ended September 30, |
Period from May 11, 1998 (inception) through September 30, 2000 |
|||||||||||||||||||||
| |
1999 |
Pro forma PPGx acquisition 1999 |
1999 |
2000 |
Pro forma PPGx acquisition 2000 |
|||||||||||||||||||
| |
(in thousands, except share and per share data) |
|||||||||||||||||||||||
Statements of Operations Data: |
||||||||||||||||||||||||
| Revenues | $ | — | $ | 79 | $ | 966 | $ | — | $ | 500 | $ | 1,672 | $ | 579 | ||||||||||
| Operating expenses: | ||||||||||||||||||||||||
| Cost of revenues | — | — | 1,030 | — | — | 1,243 | — | |||||||||||||||||
| Research and development | 20 | 4,901 | 7,022 | 3,809 | 14,602 | 17,108 | 19,523 | |||||||||||||||||
| General and administrative | 309 | 2,700 | 6,518 | 1,995 | 4,652 | 8,109 | 7,661 | |||||||||||||||||
| Stock-based compensation | — | 418 | 530 | 129 | 5,985 | 6,069 | 6,403 | |||||||||||||||||
| Amortization of samples, goodwill and acquisition related intangibles | — | — | 9,158 | — | — | 6,869 | — | |||||||||||||||||
| Total operating expenses | 329 | 8,019 | 24,258 | 5,933 | 25,239 | 39,398 | 33,587 | |||||||||||||||||
| Loss from operations | (329 | ) | (7,940 | ) | (23,292 | ) | (5,933 | ) | (24,739 | ) | (37,726 | ) | (33,008 | ) | ||||||||||
| Interest income (expense), net | (19 | ) | 103 | 20 | 85 | 1,282 | 895 | 1,366 | ||||||||||||||||
| Net loss | $ | (348 | ) | $ | (7,837 | ) | $ | (23,272 | ) | $ | (5,848 | ) | $ | (23,457 | ) | $ | (36,831 | ) | $ | (31,642 | ) | |||
| Basic and diluted net loss per share | $ | — | $ | (16.01 | ) | $ | (46.95 | ) | $ | (13.43 | ) | $ | (16.59 | ) | $ | (25.93 | ) | |||||||
| Shares used in computing basic and diluted net loss per share | — | 489,382 | 495,686 | 435,342 | 1,413,990 | 1,420,294 | ||||||||||||||||||
| Pro forma basic and diluted net loss per share | $ | (1.49 | ) | $ | (1.56 | ) | ||||||||||||||||||
| Shares used in computing pro forma basic and diluted net loss per share | 5,243,896 | 15,011,622 | ||||||||||||||||||||||
22
The balance sheet data displayed in the "Pro forma PPGx acquisition 2000" column reflects the pro forma effects of the acquisition of PPGx as if it had taken place on September 30, 2000.
| |
|
|
September 30, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
As of December 31, |
||||||||||||
| |
|
Pro Forma PPGx Acquisition 2000 |
|||||||||||
| |
1998 |
1999 |
2000 |
||||||||||
| |
(in thousands) |
||||||||||||
Balance Sheet Data: |
|||||||||||||
| Cash and cash equivalents | $ | 1,077 | $ | 3,483 | $ | 40,882 | $ | 41,234 | |||||
| Total assets | 1,150 | 8,026 | 61,453 | 98,386 | |||||||||
| Working capital (deficit) | (421 | ) | 791 | 31,148 | 29,410 | ||||||||
| Total long-term liabilities | — | 1,582 | 5,520 | 5,520 | |||||||||
| Redeemable convertible preferred stock | — | 11,345 | 69,809 | 103,820 | |||||||||
| Deferred stock compensation | — | (3,047 | ) | (15,205 | ) | (15,654 | ) | ||||||
| Total stockholders' equity (net capital deficiency) | (348 | ) | (7,750 | ) | (24,263 | ) | (23,989 | ) | |||||
23
MANAGEMENT'S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read with "Selected Financial Data" and our financial statements and notes included elsewhere in this prospectus. The discussion in this prospectus contains forward-looking statements that involve risks and uncertainties, such as statements of our plans, objectives, expectations and intentions. The cautionary statements made in this prospectus should be read as applying to all related forward-looking statements wherever they appear in this prospectus. Our actual results could differ materially from those discussed here. Factors that could cause or contribute to these differences include those discussed in "Risk Factors," as well as those discussed elsewhere.
Overview
We are a genetics discovery company focused on identifying the genetic basis of disease susceptibility, disease progression and response to drug treatment. We were incorporated in May 1998 and in May 2000 changed our name from KIVA Genetics, Inc. to DNA Sciences, Inc. For the period from our inception to December 31, 1998, we were primarily focused on fundraising and start-up activities. In 1999, we focused primarily on the development of our microchannel DNA sequencer, which incorporates technology that we licensed from University of California, Berkeley in 1998 and Amersham Pharmacia Biotech. During 1999 we also developed software to manage genetic information. Beginning in 2000, we expanded our business to focus on research and development efforts for our gene discovery programs and commenced building our genotyping and sequencing facility. We plan to develop diagnostic tests and, in the longer term, therapeutic products based on gene variants we identify through our discovery programs. Revenues from these products and services will not be realized for several years, if ever.
To date, we have derived our revenues almost entirely from a $2.0 million grant under the United States Department of Commerce's Advanced Technology Program. This grant was awarded to fund development of our microchannel DNA sequencer. Our acquisition of PPGx in December 2000 will add revenues from genotyping services performed for pharmaceutical companies, including Eli Lilly & Co., Pharmacia Corp. and Bristol Myers Squibb Company. In December 2000, we entered into a contract with Amersham Pharmacia Biotech under which we licensed our technology and intellectual property which the DNA sequencer utilizes to Amersham in exchange for, among other things, cash payments totaling $7.5 million over three years commencing in 2001.
Our operating costs and expenses consist primarily of research and development expenses, information technology development and expenses associated with our website, and general and administrative expenses. Our research and development expenses include salaries, supplies, the cost of recruiting patients and obtaining DNA samples, machine and reagent use and related facilities and information technology expenses. Patient recruitment costs include the cost of our DNA.com website, payments under our collaboration agreement with WebMD and other related promotion costs. Our general and administrative expenses include compensation related to executive, business development, finance and other administrative personnel, related facilities costs, insurance and legal support. Our total full time employees increased to 72 individuals as of September 30, 2000 from 40 as of December 31, 1999 and four as of December 31, 1998. As our headcount has increased, our operating expenses have increased accordingly.
We anticipate that our results of operations will fluctuate for the foreseeable future due to numerous factors, including the progress of our genetic discovery programs, market evaluation and acceptance of our products and services, patent requirements and the introduction of new products by our competitors. The sales cycle for genotyping services is highly variable and dependent upon the simultaneous availability of production capacity and research project demand. We expect that it may
24
take between six weeks and six months to progress from initial contact to execution of a contract services agreement.
We have a limited operating history. Since inception we have incurred significant losses. On a pro forma basis, after giving effect to our acquisition of PPGx in December 2000, we had net losses of $36.8 million for the nine months ended September 30, 2000 and $23.3 million for the year ended December 31, 1999. As of September 30, 2000, we had an accumulated deficit of $31.6 million. We anticipate incurring additional and increasing losses for many years as we expand our genetic discovery efforts, increase our genotyping production capabilities and develop commercial products and services. Our limited history and the limited history of PPGx make accurate forecasts of future operations difficult.
Deferred Stock Compensation
Deferred stock compensation represents the difference between the fair value of our common stock for financial reporting purposes and the exercise price of options on their date of grant. We are amortizing the deferred stock compensation to expense as these options vest, generally over four to five years. During the period ended December 31, 1999, we recognized stock compensation expense totaling $418,000. Additional deferred stock compensation expense totaling $6.0 million was recognized during the nine months ended September 30, 2000. As of September 30, 2000, there was approximately $15.2 million of deferred stock compensation to be amortized in future periods. In addition to deferred stock compensation related to grants of stock options to our employees and directors, we also recognize expense for the value of options and warrants granted to non-employee consultants and service providers. During the year ended December 31, 1999 and the nine months ended September 30, 2000 we recognized expense of $66,000 and $2.6 million, respectively, related to such grants. The value of grants to non-employees is measured as they are earned over their vesting periods.
In the period from September 30, 2000 through December 31, 2000 we granted employees and consultants additional stock options to purchase 2,574,790 shares of common stock at exercise prices ranging from $0.75 to $2.05 per share. We will record additional deferred stock-based compensation of approximately $22.3 million in the quarter ending December 31, 2000 to account for the difference between the exercise price of option grants to employees and the fair value for financial reporting purposes of the common stock on the date of grant. In addition, we have granted stock options and purchase rights to non-employees which will be remeasured and recognized as expense over their vesting periods.
As of December 31, 2000 total deferred stock compensation will be amortized as follows:
Acquisition of PPGx, Inc.
In December 2000, we acquired PPGx, Inc., a joint venture between Pharmaceutical Product Development, Inc., or PPD, and Axys Pharmaceuticals, Inc. PPGx was incorporated on January 25, 1999. PPGx performs genetic testing and contract research services for pharmaceutical companies. PPGx has 49 full time employees located primarily in its facilities in Research Triangle Park, North Carolina and La Jolla, California. PPGx also employs three persons in its certified laboratory in Cambridge, England. Upon the closing of the acquisition, PPGx amended and restated its distributor
25
agreement with PPD. Under this agreement, PPD was appointed the exclusive distributor of pharmacogenomic products and services developed by or licensed to PPGx, except for diagnostic kits or similar products. In exchange for providing marketing and distribution services, PPD will receive a percentage of the revenues from sales of products and services marketed by PPD and supplied or provided by PPGx.
As consideration for the acquisition, we issued 2,957,100 shares of our Series D preferred stock and 6,304 shares of common stock. As part of the purchase price, we also assumed options to purchase approximately 1,405,900 shares of common stock of PPGx which were converted into options to purchase approximately 305,800 shares of our common stock outside our stock plans. We will account for the acquisition using the purchase method of accounting. Based on a preliminary valuation, we will allocate the excess of the purchase price over the fair value of the net assets acquired as follows:
| In process research and development | $ | 1,700,000 | |
| Assembled workforce | 1,100,000 | ||
| Developed and core technology | 6,500,000 | ||
| DNA samples | 15,600,000 | ||
| Goodwill and other intangibles | 10,540,000 |
We expect the intangibles to be amortized over periods ranging from two to five years. In addition, the financial information does not include any costs related to the integration of PPGx, which have not yet been determined. For more information, see the "Unaudited Pro Forma Combined Condensed Financial Statements" included elsewhere in this prospectus.
PPGx has derived its revenues primarily from contract research and genetic testing services. Such amounts were recorded after revenue sharing in accordance with a prior agreement with PPD. For the nine months ended September 30, 2000, PPGx had total revenues of $1.2 million and a net loss of $6.4 million. For the period from January 25, 1999 (inception) to December 31, 1999, PPGx had total revenues of $887,000 and a net loss of $6.2 million. Sales to PPD for services provided and products sold to Eli Lilly, Pharmacia and Bristol-Myers Squibb accounted for approximately 30%, 18% and 8% respectively of PPGx's revenues in the nine months ended September 30, 2000. Revenues from services provided and products sold directly to PPD accounted for 10% of PPGx's total revenues in the period. No other customer accounted for more than 7% of PPGx's total revenues.
PPGx's operating costs and expenses consist primarily of costs incurred for contract research and genetic testing services, internal research efforts and general and administrative expenses. Cost of revenues include salaries of scientific personnel and costs of supplies related to providing research and testing services. PPGx's research and development expenses were approximately $2.5 million for the nine months ended September 30, 2000 and approximately $2.1 million for the period from January 25, 1999 to December 31, 1999. General and administrative expenses consist primarily of facility expenses and executive, finance and administrative personnel costs. PPGx's general and administrative expenses were approximately $3.5 million for the nine months ended September 30, 2000 and approximately $3.8 million for the period from January 25, 1999 to December 31, 1999.
Results Of Operations
Nine months ended September 30, 2000 and 1999
Revenues
Total revenues were approximately $500,000 for the nine months ended September 30, 2000. We did not recognize revenues in the nine months ended September 30, 1999. The increase was entirely attributable to revenue recognized under our research grant from the Department of Commerce's Advanced Technology Program.
26
Research and development expenses
Our research and development expenses increased to approximately $14.6 million for the nine months ended September 30, 2000 from approximately $3.8 million for the same period in 1999. The increase was attributable to the operation of our significantly expanded genotyping production facility, development of our microchannel DNA sequencer, recruitment of patients into our Gene Trust project, including the cost of our collaboration with WebMD, as well as increased hiring of research staff. We expect our research and development costs to increase substantially as we expand our genetic discovery efforts, expand the use of our website to gain access to patient samples and develop commercial products based on our discoveries.
General and administrative expenses
Our general and administrative expenses increased to approximately $4.7 million for the nine months ended September 30, 2000 from approximately $2.0 million for the same period in 1999. This increase was attributable to increased business development efforts, increased headcount and legal costs. We expect our general and administrative expenses to increase substantially as we expand our infrastructure to support our anticipated increased operations and integrate PPGx.
Stock compensation expenses
Stock compensation expenses associated with the issuance of stock options to our employees and consultants increased to approximately $6.0 million for the nine months ended September 30, 2000 from $129,000 for the same period of 1999. The amount allocable to research and development personnel was approximately $3.0 million and the amount allocable to general and administrative personnel was also approximately $3.0 million in the nine months ended September 30, 2000.
Interest income (expense), net
Our interest income (expense), net increased to approximately $1.3 million for the nine months ended September 30, 2000 from approximately $85,000 for the same period in 1999. This increase was primarily attributable to an increase in cash balances resulting from the sale of preferred stock.
Period from May 11, 1998 (inception) to December 31, 1998 compared to Year ended December 31, 1999
Revenues
Total revenues were approximately $79,000 for the year ended December 31, 1999. There were no revenues in the period from May 11, 1998 (inception) to December 31, 1998. Revenues in 1999 were attributable to funds received under our research grant from the Department of Commerce's Advanced Technology Program.
Research and development expenses
Research and development expenses increased to approximately $4.9 million for the year ended December 31, 1999 from approximately $20,000 for the period from inception to December 31, 1998. This increase was attributable to the acquisition and build out of the research and development portion of our initial facility, an increase in research and development headcount and development costs related to our microchannel DNA sequencer.
General and administrative expenses
Our general and administrative expenses include compensation expenses related to executive, business development, finance and other administrative personnel, the related costs of our facilities and insurance and legal support. Our general and administrative expenses increased to approximately
27
$2.7 million for the year ended December 31, 1999 from approximately $309,000 for the period from inception to December 31, 1998. The increase was attributable to an increase in employees, business development efforts, management hiring, and legal costs.
Stock compensation expenses
Stock compensation expenses associated with the issuance of stock options to our employees and consultants was $418,000 for the year ended December 31, 1999. We did not recognize any stock compensation expenses in 1998. The amount allocable to research and development personnel was approximately $239,000 and the amount allocable to general and administrative personnel was $179,000 in 1999.
Interest income (expense), net
Interest income (expense), net increased to approximately $103,000 for the year ended December 31, 1999 from approximately ($19,000) for the period from inception to December 31, 1998.
Provision for income taxes
As of December 31, 1999, we had approximately $6.4 million of federal and $6.3 million of California state net operating loss carryforwards for tax reporting purposes to offset future taxable income. We also had research and development tax credits of approximately $100,000 for federal income tax purposes. The net operating loss carryforwards will expire at various dates beginning in 2006 through 2019, if not utilized. We have not recognized any benefit from the future use of carryforwards since inception because of uncertainty regarding their realization. The amount of net operating loss that we can utilize may be limited under tax regulations in future periods. Utilization of the net operating losses and credits may be subject to a substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986 and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization. We have not calculated the amount of the limitation, if any.
Liquidity and Capital Resources
We have financed our operations from inception through private sales of preferred stock, equipment financing arrangements and funds received under our research grant from the Department of Commerce's Advanced Technology Program. Through September 30, 2000, we had received net proceeds of $67.1 million from the issuance of common and preferred stock and $579,000 from our research grant. In addition, through September 30, 2000, we received proceeds of $3.0 million from equipment financing arrangements. These obligations are secured by the equipment financed, bear interest at the rates between 13% to 14% per year and are due in monthly installments through 2003. In December 2000, we received net cash proceeds of $31.5 million from the sale of additional shares of preferred stock.
As of September 30, 2000 we had $40.9 million in cash, cash equivalents and short-term investments. For the nine months ended September 30, 2000, we used $11.0 million in cash for operating activities. This consisted of the net loss for the period of $23.5 million offset in part by non-cash charges of $8.1 million, which included stock compensation expenses of $6.0 million and depreciation and amortization expenses of $2.1 million. During 1999, we used $5.2 million for operating activities. In 1999 this consisted of the net loss for the period of $7.8 million offset in part by non-cash charges of $2.1 million, which included stock compensation expenses of $418,000, depreciation and amortization charges of $373,000 and $1.3 million related to the acquisition of technology rights. In the period from our inception to December 31, 1998 we used approximately $243,000 in cash for operating activities.
28
For the nine months ended September 30, 2000, net cash used in investing activities was $9.7 million for capital expenditures including approximately $1.4 million for machinery and equipment and $7.2 million for computers and computer software. We also financed directly the acquisition of $6.6 million of equipment through indebtedness repayable over 32 months. In 1999, net cash used in investing activities was $3.2 million for capital expenditures primarily machinery and equipment. In the period from our inception to December 31, 1998, net cash used in investing activities was immaterial.
Net cash provided by financing activities was approximately $58.2 million for the nine months ended September 30, 2000, $10.7 million in 1999 and $1.4 million in 1998. Our financing activities primarily consisted of sales of our preferred stock and common stock and proceeds from equipment financings.
In the period from September 30, 2000 through December 31, 2000 we entered into two separate credit facilities for up to an aggregate of $8.0 million of financing for the purchase of capital equipment, of which $1.8 million was available as of December 31, 2000. These obligations are secured by the equipment financed, bear interest at rates between 13% and 14% per year and are due in monthly installments through 2003.
We have cash commitments under various agreements, including:
We expect to have negative cash flow for the foreseeable future. We expect to spend significant amounts over the next several years to discover disease-associated gene variants, further develop and commercialize our diagnostic tests, upgrade our production genotyping and informatics capabilities, improve and expand our facilities, complete the integration of PPGx and attract and retain skilled research, marketing and administrative personnel. Our future capital requirements will depend on a number of factors, including the number and progress of our genetic discovery programs, revenue derived from genotyping services, the cost of maintaining any strategic relationships, such as our relationships with WebMD and the University of Utah, and the cost of acquiring access to new technologies and genomic databases. We believe that our current cash balances, together with the net proceeds of this offering and revenues to be derived from additional research services, collaborative research studies, and genetic testing services will be sufficient to fund our operations through at least 2002. For a discussion of the risks to our business if we are unable to satisfy our future capital needs see "Risk Factors—We have a history of losses and negative cash flows and anticipate continued losses for the foreseeable future."
Quantitative and Qualitative Disclosures about Market Risk
Our cash equivalents and short-term investments are exposed to financial market risk due to fluctuations in interest rates, which may affect our interest income and, in the future, the fair market value of our investments. We manage our exposure to financial market risk by performing ongoing evaluations of our investment portfolio. We presently invest in highly liquid, investment grade securities. These securities are highly liquid and mature no more than 90 days from our purchase date. Due to the short maturities of our investments, the carrying value should approximate the fair value. In addition, we do not use our investments for trading or other speculative purposes. As our cash balances increase, we anticipate continuing to invest in these same types of securities. We have performed an analysis to assess the potential effects of reasonably possible near term changes in interest. Due to the
29
short duration of our investment portfolio, an immediate 10% change in interest rates would not have a material effect on the fair market value of our portfolio. Therefore, we would not expect our operating results or cash flow to be affected to any significant degree by the effect of a sudden change in market interest rates on our securities portfolio.
Recent Accounting Pronouncements
In June 1998, the Financial Accounting Standards Board, or FASB, issued Statement of Financial Accounting Standards No. 133, "Accounting for Derivative Instruments and Hedging Activities". SFAS 133 establishes accounting and reporting standards for derivative instruments, including certain derivative instruments embedded in other contracts, and for hedging activities. SFAS 133, as amended by SFAS 137, is effective for all fiscal quarters of all fiscal years beginning after June 15, 2000, with earlier application encouraged. We do not currently use derivative instruments and therefore do not expect that the adoption of SFAS 133 will have any impact on our financial position or results of operations.
In December 1999, the SEC issued Staff Accounting Bulletin No. 101, "Revenue Recognition in Financial Statements". SAB 101 summarizes the SEC's views in applying generally accepted accounting principles to revenue recognition. The adoption of SAB 101 had no impact on our historical revenue recognition policy.
In March 2000, the FASB issued FASB Interpretation No. 44, "Accounting for Certain Transactions Involving Stock Compensation", which contains rules designed to clarify the application of Accounting Principles Board Opinion No. 25. FIN 44 was effective on July 1, 2000. The adoption of FIN 44 did not have a material effect on our operating results and financial position.
30
Background
The Human Genome
Each cell contains the entire genetic code of an organism in molecules of DNA. DNA is a chemical chain consisting of two strands of chemical units called nucleotide bases. These strands consist of four different types of nucleotide bases, with each commonly known by the first initial of its name: G, C, A and T. The order in which these bases appear in the chemical chain is called the DNA sequence. The complete DNA of an organism is referred to as its genome.
Genes are segments of DNA that encode instructions for the cell to produce proteins. There are estimated to be between 50,000 to 100,000 genes in the human genome. Each cell contains a full set of genes, but expresses only those genes necessary for its specific function. For example, insulin is only made by pancreatic cells. Each protein carries out a different function. The correct proteins must be produced at the right time, in the correct cells and in the right amounts for the body to function properly.
DNA variants in the human genome make each person distinct. These variants contribute to everything from hair and eye color to susceptibility to a particular disease or drug response. The most abundant class of genetic variants in the human genome are called single nucleotide polymorphisms, or SNPs, which involve a change in only one base of the DNA. SNPs occur on average once every 250 to 1,000 nucleotide bases within the genome. There are an estimated 10 to 30 million different SNPs collectively among the genomes of the human population and up to one million SNPs in any given individual. Although most SNPs have no apparent medical significance, certain SNPs can have an enormous impact on human biology. For example, one SNP in the beta-hemoglobin gene causes sickle cell anemia if inherited from both parents. This SNP bestows relative immunity from malaria in those who inherit the variant from only one parent.
Short tandem repeats, or STRs, are another important class of genetic variants. STRs are pieces of DNA that consist of groups of two to four nucleotide bases that are repeated one to ten times and are surrounded by unique DNA sequence. Related individuals are more likely to share a greater number of specific STRs than unrelated individuals. Specific STRs mark or define a specific location in the genome. STRs define a larger portion of the genome than SNPs and therefore are more likely than SNPs to be on the same portion of DNA as the gene variant responsible for a disease. Although more than 5,000 STR markers have been identified, fewer than 1,000 are necessary to interrogate the entire genome.
In the early 1990s, the Human Genome Project, a collective effort supported by various governmental and private sources, was undertaken to establish the complete DNA sequence of humans. Commercial entities are also attempting to sequence the entire human genome. A draft of the human genome sequence was announced in June 2000. Although this sequencing information is useful for basic research, it does not explain the role gene variants play in causing disease.
Genetics, Penetrance and Disease
Genetics is the study of genetic variations in individuals and how those variations are passed down from one generation to the next. Genetic research designed to discover the relationship between gene variants and disease requires quantitative analyses that compare variations in DNA sequence found among persons with the disease to those without the disease. The results of genetic analyses are expressed in terms of probability. For example, there is a one in four chance that each child of parents who both have the cystic fibrosis gene-variant will inherit that gene variant. If a child does inherit the gene variant from each parent, it is certain the child will develop cystic fibrosis.
31
Gene variants play diverse roles in causing disease. Some genetic diseases are clearly passed down from one generation to the next. Such diseases usually involve a rare gene variant called a mutation that severely affects the function of a single gene and causes the disease. Cystic fibrosis, sickle cell anemia and Huntington's disease are examples of diseases caused by rare mutations in single genes. However, in many common diseases, such as diabetes, asthma, cardiovascular disease and cancer, gene variants create susceptibility to the disease but do not alone cause it. In addition to genetic variants, non-genetic factors such as diet and lifestyle may contribute to susceptibility to these diseases. There are also rare causes of some common diseases that are the result of mutations in a single gene. For example, a rare form of breast cancer is caused by mutations in the BRCA1 and BRCA2 genes. While this form of the disease is clinically indistinguishable from more common forms, less than 5% of all breast cancer cases can be attributed to mutations in these genes. The gene variants that account for the majority of the genetic susceptibility to breast cancer have not yet been found.
For a population of individuals with a particular gene variant, penetrance defines the proportion of individuals that carry the variant that will actually be diagnosed with the specific disease. Fully penetrant gene variants always cause a particular disease or condition. Other gene variants, however, only increase the risk of developing the specific disease and are said to be partially penetrant. We believe that much of the genetic basis of the majority of common diseases is caused by moderately penetrant gene variants that are present in a small percentage of the population.
Current Approaches to Identification of Genes Contributing to Disease
Current approaches to the identification of genes causing disease generally fall into two categories: classical linkage analysis and recently initiated case-controlled whole genome SNP association studies.
Linkage Analysis
Linkage analysis is an approach that involves linking relevant genetic markers to a specific condition by analyzing families in which the markers and the condition can be traced through successive generations. Using this approach, gene variants that cause a particular disease are identified by associating the presence of the disease in an individual with the presence of a specific STR marker in that individual. Because STR markers are found throughout the genome, any given gene variant responsible for a disease is likely to be close to an STR marker. If a given STR marker is always present among relatives with a specific condition, there is a high probability that the disease-associated gene variant is near that STR marker.
This method has several limitations for the discovery of moderately penetrant gene variants. First, the discovery of moderately penetrant gene variants using this method requires studies of very large families in order to reach statistical significance. These studies are expensive and often not feasible. Second, multiple genetic causes complicate linkage analysis because different families may have different gene variants that cause the disease. Third, linkage analysis studies are constrained by the need for more than one generation of affected individuals. For example, studying diseases that occur late in life is more difficult because parents of affected individuals may be dead. For these reasons, linkage analysis is best suited for studies of families where a single, highly penetrant gene variant, present in all the families, is responsible for the disease. Linkage analysis is less suited for the study of moderately penetrant genes associated with common diseases.
Case-Controlled Whole Genome SNP Association Studies
A recently proposed alternative approach to identifying disease-associated gene variants is referred to as a case-controlled whole genome SNP association study. Unlike linkage analysis, this method uses SNPs, not STRs. This approach has received considerable attention as a result of a recent substantial increase in the number of discovered SNPs and the development of genotyping technology such as
32
DNA chips and mass spectroscopy. In case-controlled whole genome SNP association studies, unrelated individuals affected with a condition are compared to unaffected individuals to determine which SNPs are shared by the affected individuals.
Case-controlled whole genome SNP association studies are potentially useful for identifying disease-associated gene variants causing disease but have significant limitations. First, it has been estimated that up to 500,000 SNP assays may be needed to interrogate the entire genome of a single person because of the small amount of genetic information that can be derived from each SNP. Furthermore, each study may require thousands of participants. Currently, the cost to reliably assay a single SNP in an individual is just under $1.00 per SNP. In addition, most of these newly discovered SNPs have yet to be verified and converted into reliable assays, a process that can cost up to $100 per SNP. As a result, this approach can be extremely expensive.
Second, most of these newly discovered SNPs may occur too frequently to be responsible for genetic susceptibility to common diseases or useful in detecting the presence of the responsible SNPs. For example, the lifetime risk for breast cancer in the United States female population is one in nine. Approximately 30% of these breast cancer cases can be attributed to a hereditary susceptibility to the disease. We believe that it is likely that breast cancer in a given susceptible family is caused by a moderately penetrant, relatively uncommon gene variant or variants each present in 0.1% to 2% of the female population. In order to be comprehensive, methods such as SNP association must include the analysis of relatively uncommon SNPs to increase the chances of finding the disease-associated gene variants. Most low frequency SNPs have yet to be identified.
Third, case-controlled whole genome SNP association studies use groups of unrelated individuals affected with a particular disease. These groups include a high proportion of patients who may have the disease for reasons other than genetic susceptibility. For example, in the case of breast cancer, approximately 700 out of 1,000 women with the disease will have developed it without an apparent genetic cause. The high proportion of non-genetic cases reduces the sensitivity of the approach for finding the genetic associations. While the problem can be overcome by using tens of thousands of patients, this is impractical for most studies because of cost and time.
Fourth, large amounts of SNP genome sampling result in many more false positive associations than true positive associations between specific variants and a particular disease. For example, if a SNP study looks at 500,000 SNPs per individual, there may be thousands of false positive associations per study. All positive associations, whether false or real, need to be validated in a separate study testing for the association in a new population. This retesting can be prohibitively time consuming and expensive.
Our Approach
We use multiple methods to identify and validate disease-associated gene variants. The method we select depends on a variety of factors, including the suspected penetrance of the gene variants we seek, the availability of DNA samples and the extent to which prior genetic studies have implicated specific regions of the genome. Once a candidate region has been identified, either through our efforts or those of a third party, we begin the process of identifying all genes and their variants in that region. Where a search of public and private databases yields no gene variants in that candidate region, we will deep sequence that region to find new gene variants. Deep sequencing is the determination of DNA sequence including variants in a specific gene or region in a large number of people with the specific goal of indentifying new gene variants that are relatively uncommon. We will validate our findings by confirming the association between the identified gene variant and the disease in a large and geographically dispersed population of unrelated individuals obtained through the Gene Trust project.
33
The following chart illustrates how we combine our methods to discover and validate disease-associated gene variants:
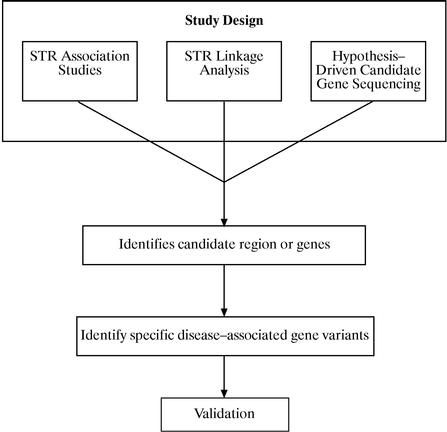
Study Design
We employ a variety of study designs to identify gene variants associated with diseases and response to treatment. These study designs include STR association studies, STR linkage analysis and hypothesis-driven candidate gene sequencing. Each study design requires different patient groups and genome analytic methods. In order to identify moderately penetrant gene variants we undertake STR association studies, involving large kinships principally comprised of members of the same generation with a disproportionate occurrence of the disease under study. In order to identify highly penetrant gene variants we use STR linkage analysis, which requires multi-generational families with a recognizable pattern of inheritance of the disease under study. Hypothesis-driven candidate gene sequencing requires sequencing specific sites in the genome of unrelated affected individuals. We believe that by using multiple study designs, we significantly improve the probability of identifying gene variants with varying degrees of penetrance.
STR Association Studies
STR association studies involve the search for a disease-associated gene variant in large groups of distantly related individuals, known as kinships, which have a disproportionate number of cases of a particular disease. This study design provides us with three distinct advantages. First, the affected members of these kinships are more likely to have a genetic basis for their condition than a randomly selected group of affected individuals for whom most will not have a genetic cause. Second, each affected individual within the kinship is likely to have a single, identical gene variant causing the condition. This reduces the complexity of the genetic analysis in each kinship. A single, identical disease-associated gene variant in a given kinship is easier to discover than several gene variants. Third,
34
kinships can be selected on the basis of how many cases they have relative to the general population. This allows us to quantify the genetic susceptibility within the kinship and the average clinical risk of disease in a person with the particular gene variant. For example, if a given kinship has a three-fold excess prevalence of breast cancer cases, the genetic variants that are discovered in that kinship should confer on average a three-fold greater risk to each individual who carries that variant. Thus, the clinical utility of a given gene variant is determined by the selection of specific affected kinships at the beginning of the process, not discovered after the study is completed.
STR Linkage Analysis
STR linkage analysis is the traditional method of identifying disease-causing genes in families. Prior linkage analysis performed on various populations has identified regions of the genome associated with disease. In most studies, however, the precise location of the disease-causing gene has not yet been identified. This is because, until recently, only a small portion of the human genome had been mapped. In addition, the amount of sequencing required to precisely locate a disease-associated gene has historically been prohibitively expensive. Given the availability of the nearly complete human genome sequence, the localization of these disease-associated gene variants will proceed much more quickly. We have focused our efforts on obtaining rights to linkage studies where data and samples can be interrogated in combination with genome maps and sequence obtained from public and proprietary sources. To date, we have acquired from PPGx rights to linkage analysis data and DNA samples from patients with asthma, osteoporosis, inflammatory bowel disease and diabetes. We will continue to explore the opportunity to obtain DNA samples and linkage analysis data on an ongoing basis.
Hypothesis-Driven Candidate Gene Sequencing
The extensive understanding of a specific disease sometimes suggests the involvement of a specific gene or family of genes that might contribute to the disease. Additionally, an understanding of one disease sometimes provides clues about the mechanisms of another disease. For example, the genetic form of the long QT syndrome, a cause of sudden death in adults, has been definitively linked to five separate genes using STR linkage analysis. This form of sudden death in adults bears a strong clinical resemblance to SIDS, which suggests that some cases of SIDS may be caused by gene variants in these same five genes. We are currently exploring that hypothesis by sequencing all five genes in 120 postmortem samples from patients with a diagnosis of SIDS. We will extend these sequencing studies to investigate whether other variants of these five genes are also associated with the more common forms of cardiac arrhythmias that cause sudden death.
DNA Study Tools
We use a variety of tools to analyze DNA. These include STR markers, SNP genotyping and deep sequencing.
STR Markers
We use commercially available and scientifically validated STR markers to identify regions in the genome containing disease-associated gene variants. Each STR marker defines a specific site in the genome. STR markers contain a specific number of nucleotide repeats that vary from individual to individual. By using STR markers distributed across the genome, we can interrogate the entire genome with fewer than 1,000 markers. In contrast, up to 500,000 SNP markers would be needed to scan the whole human genome. As a result, an STR genome scan is cheaper and faster and may be more likely to identify the relevant region of the genome than a comparable SNP genome scan. For a given study, STR genotypes are performed in all affected individuals and a comparable number of unaffected individuals. STR genotyping is the first step in narrowing the search for disease-associated gene variants to specific regions of the genome.
35
SNP Genotyping
In candidate regions of the genome, we will identify SNPs using public and proprietary genomic sources, such as The SNP Consortium, as well as through our own sequencing of those regions. SNPs, like STRs, serve as markers in the genome that provide a more detailed view of a smaller region of the genome than STRs. As a result, an SNP marker may be much closer physically to a particular disease-causing gene variant than an STR marker. By identifying SNPs in candidate regions, we are able to narrow our search for the disease-associated gene variant to an area small enough to allow deep sequencing in a large number of affected and unaffected individuals.
Deep Sequencing
Deep sequencing is the determination of the DNA sequence in a specific gene or region in a large number of people. Deep sequencing allows the identification of novel gene variants, such as SNPs, that can then be used to further localize the disease-associated gene variants. This is accomplished by SNP genotyping the same regions that were first identified by STR markers. In order to strengthen our intellectual property position and enhance clinical utility, we seek to find all gene variants in that region of the genome that cause a particular disease as well as gene variants that are always found in conjunction with the disease-causing gene variants. Deep sequencing of a large and diverse population of affected individuals also allows us to validate our discovery of the disease-associated gene variants.
Study Validation
Once we have identified a disease-associated gene variant using a study design that relies on related individuals, we will seek to confirm the association between the identified gene variant and the disease in a large and diverse population of unrelated individuals. We may accomplish this using patients recruited through the Gene Trust. We believe that Gene Trust volunteers represent a broad cross-section of the U.S. population. Such a heterogeneous population is ideal for validating and extending results of studies based on patient populations derived from relatively restricted geographic or ethnic origins. After securing intellectual property rights, we will make public the results of our studies to encourage independent third parties to replicate and further validate our results.
Patient Recruitment
We are using multiple approaches to recruit patients and obtain DNA samples. One approach is to identify kinships using the Utah Population Database and determine the prevalence of disease in those kinships. Another approach is to obtain DNA samples, candidate genes and data derived from family-based linkage analyses performed by third parties. Once we have preliminarily identified disease-associated gene variants, we intend to validate our findings in a group of unrelated individuals using our Gene Trust volunteers. In addition, we may use our Gene Trust project to develop patient populations for identifying disease-associated genes.
Kinships
Our sponsored research at the University of Utah allows us to collaborate with the University's faculty members to conduct genetic studies which utilize, on a non-exclusive basis, the Utah Population Database. This database represents the largest source of patients for our studies. The Utah Population Database traces the genealogy of 50,000 pioneer couples who immigrated to Utah in the 19th century. Their descendants number more than 1.5 million residents of Utah for whom genealogies have been determined. This database is a unique resource for geneticists because of the large family size, complete family records and a historical willingness of the Utah residents to participate in genetic studies. As a result, there are very few other databases in the world that are as useful to genetic studies. Because this database is electronic, our collaborators have the ability to rapidly identify
36
numerous large kinships suitable for our study design. The Utah Population Database is primarily comprised of descendants from many regions of Europe and therefore is representative of much of the U.S. population. We believe that this ensures our results will be widely applicable to the general U.S. population. We have exclusive consulting relationships with our current collaborators at the University of Utah Medical Center.
Gain Access to Results of Completed Family-Based Linkage Studies
We will continue to seek access to DNA samples, gene candidates and data where family-based linkage studies have already been performed. To date, we have obtained rights to samples in a variety of disease areas through our acquisition of PPGx, candidate genes for SIDS from an academic institution and tissue samples from individuals with SIDS from a National Institutes of Health-funded tissue bank. In most cases, including all of the samples obtained through PPGx, candidate regions containing disease-associated gene variants have already been identified. We intend to continue to pursue this strategy of obtaining these assets from outside sources.
Validate in Broader Population through the Gene Trust Project
We intend to validate our discoveries of disease-associated gene variants through study of unrelated patients recruited from the Gene Trust project. We established the Gene Trust to recruit volunteers over the Internet into our genetic studies. Out of more than 250,000 visits to our website as of December 1, 2000, more than 5,000 persons have agreed to participate in the project. In order to increase traffic to our website, we have partnered with WebMD, a leading healthcare information website with over 500,000 users. For a discussion of our relationship with WebMD, see "—Strategic Relationships" and "Related Party Transactions."
Microchannel DNA Sequencer
We have developed a prototype microchannel DNA sequencer that accelerates the ability to obtain DNA sequence information from patient samples. Our microchannel DNA sequencer consists of an automated, proprietary DNA sample loader, a sample injection mechanism on a disc containing 96 small glass etched channels called capillaries and a laser-based optical reader. Using the automated, proprietary loader, samples of fluorescently-labeled DNA are transferred from a standard 96 well plate onto the microchannel plate. Picoliter volumes, or the equivalent of several trillionths of a liter, of the sample DNA are transferred into individual six-centimeter separation channels. The DNA samples are then transported through separate channels using electricity to separate the DNA into its component DNA fragments. A rotating laser reader captures fluorescent emissions from the passing DNA fragments. Because DNA fragments migrate through the channel based on their size, the device is able to determine the sequence of a DNA segment based on the time each component fragment is read by the scanner. Because far fewer DNA molecules enter the separation channel than in conventional DNA sequencing machines, the device is able to obtain sequence data using a shorter separation channel. Because the separation channel is shorter, the sequencing run times are reduced by a factor of five.
We have licensed this technology and entered into an agreement with Amersham Pharmacia Biotech to further develop this instrument for commercial distribution in exchange for cash payments, research support, royalties, reagents at reduced cost and early access to the microchannel DNA sequencer. We believe this product will be available to us beginning in late 2001 and may eventually replace our MegaBACE devices.
37
Strategy
Our goal is to become the world's leading genetics discovery company by identifying the genetic basis of disease susceptibility, disease progress and appropriate treatment. We intend to accomplish this goal by continuing to implement the following strategies:
PPGx
In December 2000, we entered into an agreement to acquire PPGx, a joint venture between Pharmaceutical Product Development, Inc., or PPD, and Axys Pharmaceuticals, Inc. in exchange for approximately 2,957,100 shares of our Series D preferred stock and 6,304 shares of common stock. PPGx performs genetic testing and contract research services for pharmaceutical companies and has 52 employees located in five facilities. Through this acquisition, we have rights to DNA samples and genotype data from over 21,500 patients, a CLIA certified laboratory facility and a government certified facility in England. We intend to use these samples in our studies of asthma, inflammatory bowel disease, osteoporosis and diabetes. Most of these samples were originally obtained by Sequana Therapeutics, which was later acquired by Axys. The samples were obtained under a large number of
38
transfer agreements with third parties, each of which may contain limitations on the use of the samples or data derived from use of the samples. These third parties may contractually retain rights to these samples, and we may be required to obtain licenses from or make payments to these third parties to commercialize products or services based on these samples. In addition, our rights to use some of the samples and derivative data obtained from these samples may be terminated.
Upon the closing of the acquisition, PPGx amended and restated its distributor agreement with PPD. Under this agreement, PPD was appointed the exclusive, worldwide distributor of pharmacogenomic products and services developed by or licensed to PPGx, except for diagnostic kits or similar products. PPD is not permitted to market or distribute third party products or services which are substantially similar to the products and services for which PPD is the exclusive distributor. In exchange for providing marketing and distribution services, PPD will receive:
PPD has the right to initially determine the sales prices of all goods and services sold or provided by PPGx through PPD. We have the right to use PPGx's facilities, technology, products and services for our own internal research and development purposes. In addition, PPGx retains all rights, either alone or with us or some other designated party, to develop, market and sell clinical diagnostic testing services directly to physicians, patients and hospitals for the purpose of determining a patient's susceptibility to disease, disease progress and response to therapeutics. This agreement has a ten year term.
For a further discussion of our acquisition of PPGx, see "Management's Discsussion and Analysis of Financial Condition and Results of Operations—Acquisition of PPGx."
Products and Services
Products in Development
We intend to commercialize our discoveries of gene variants by developing proprietary diagnostic tests and, where possible, therapeutic products. We expect our diagnostic tests will allow patients and healthcare providers to:
39
The following table outlines the status of the current programs and the patient and genotyping requirements for the next step of each of our current programs. Each individual study may, however, significantly deviate from these requirements.
| Development programs |
Status |
Next step |
Approximate number of patients for next step |
|||
|---|---|---|---|---|---|---|
| SIDS | Genes identified | SNP genotyping and gene sequencing | 120 | |||
Colon cancer |
Recruiting patients |
STR genotyping |
1,000 |
|||
Breast cancer |
Recruiting patients |
STR genotyping |
1,000 |
|||
Asthma |
Linkage established*; genome region localized |
Identify genes in linkage regions |
1,000 |
|||
Inflammatory bowel disease |
Linkage established*; genome region localized |
Identify genes in linkage regions |
1,000 |
|||
Osteoporosis |
Linkage established*; genome region localized |
Identify genes in linkage regions |
1,000 |
|||
Type II diabetes |
Linkage established*; genome region localized |
Identify genes in linkage regions |
1,000 |
Our most advanced program is a study to identify the genetic basis for SIDS. We are also seeking to identify gene variants associated with a group of cancers, asthma, inflammatory bowel disease, osteoporosis and type II diabetes.
SIDS
The goal of our SIDS study is to develop a diagnostic product that identifies infants at risk for SIDS. There is strong clinical evidence to implicate cardiac arrhythmias as one of the more important causes of SIDS. We recently in-licensed 152 variants in a group of five LQT genes associated with a rare form of irregular heartbeat, or arrhythmia, called ventricular tachycardia that causes sudden death. We believe that these variants in one or more of the five LQT genes are responsible for this syndrome. We are currently examining 120 postmortem samples from infants with confirmed diagnoses of SIDS. We are genotyping all five LQT genes in each sample for gene variants. If we find an association between a gene variant and SIDS, we will seek a similar number of additional SIDS samples to confirm the association. In addition we will participate in prospective studies which may involve thousands of infants to determine whether testing for the presence of these variants and managing the patient accordingly can reduce mortality from SIDS.
Cancer
One of our collaborators at the University of Utah has identified kinships using the Utah Population Database and the Utah Cancer Registry in which there is a disproportionate number of cases of colon and breast cancer compared to the general population. Through this collaborator, we will collect blood samples from affected individuals in these kinships and analyze them for cancer susceptibility genes. We are in the process of obtaining institutional review board approval from the University of Utah for this study. We intend to employ a similar strategy to study genetic susceptibility for prostate cancer and melanoma which, like colon and breast cancer, have a significant genetic component. Each study may require up to 1,000 patients from 20 kinships. Additionally, validation in a
40
general population of cancer patients may require up to 1,000 patients to validate the initial findings. Some of these patients may be obtained through the Gene Trust.
Asthma
Through our acquisition of PPGx in December 2000, we obtained DNA samples and genotype data from more than 4,800 patients with asthma. Regions of the genome that may contain asthma-causing gene variants have been identified using these samples. We will seek to narrow these regions by identifying SNPs within them and then determine if they are associated with the disease. We will perform deep sequencing within these regions using the same patient samples to identify any gene variants associated with asthma. We will then validate the disease association of these gene variants by testing a broader population of asthma patients who are not genetically related. We will solicit patients for these validation studies using Gene Trust volunteers with a history of asthma who will be asked to provide DNA samples for these studies. Validation in this general population of asthma patients may require up to 1,000 patients.
Inflammatory Bowel Disease
Through our acquisition of PPGx, we obtained DNA samples and genotype data from more than 7,100 patients with inflammatory bowel disease. Regions of the genome that may contain gene variants that cause inflammatory bowel disease have been identified using these samples. We will seek to narrow these regions by identifying SNPs within them and then determine if they are associated with the disease. We will perform deep sequencing within these regions using the same patient samples to identify any gene variants associated with inflammatory bowel disease. We will then validate the disease association of these gene variants by testing a broader population of inflammatory bowel disease patients who are not genetically related. We will solicit patients for these validation studies using Gene Trust volunteers with a history of inflammatory bowel disease who will be asked to provide DNA samples for these studies. Validation in this general population of inflammatory bowel disease patients may require up to 1,000 patients.
Osteoporosis
Through our acquisition of PPGx, we have obtained DNA samples and genotype data from more than 2,400 patients with osteoporosis. Regions of the genome that may contain osteoporosis-causing gene variants have been identified using these samples. We will seek to narrow these regions by identifying SNPs within them and then determine if they are associated with the disease. We will perform deep sequencing within these regions using the same patient samples to identify any gene variants associated with osteoporosis. We will then validate the disease association of these gene variants by testing a broader population of osteoporosis patients who are not genetically related. We will solicit patients for these validation studies using Gene Trust volunteers with a history of osteoporosis who will be asked to provide DNA samples for these studies. Validation in this general population of osteoporosis patients may require up to 1,000 patients.
Type II Diabetes
Through our acquisition of PPGx, we have obtained DNA samples and genotype data from more than 2,700 patients with type II diabetes. Regions of the genome that may contain diabetes-causing gene variants have been identified using these samples. We will seek to narrow these regions by identifying SNPs within them and then determine if they are associated with the disease. We will perform deep sequencing within these regions using the same patient samples to identify any gene variants associated with type II diabetes. We will then validate the disease association of these gene variants by testing a broader population of type II diabetes patients who are not genetically related. We will solicit patients for these validation studies using Gene Trust volunteers with a history of type II
41
diabetes who will be asked to provide DNA samples for these studies. Validation in this general population of type II diabetes patients may require up to 1,000 patients.
Services
Pharmacogenetic Testing
DNA Sciences offers genotyping services directly to customers, including pharmaceutical companies. To date, DNA Sciences has entered into one agreement to provide these services to Novartis Pharmaceuticals Corporation. In addition, pursuant to its agreement with PPD, PPGx provides genotyping services to pharmaceutical customers of PPD, including Eli Lilly, Pharmacia and Bristol Myers Squibb. These services are primarily focused on gene variants that influence drug metabolism. We intend to expand our pharmacogenetic services by leveraging PPD's relationships with pharmaceutical companies and offering a broader array of pharmacogenetic tests.
Clinical Testing
We intend to use our CLIA-certified facility to offer specific genetic tests for clinical use. The first test we expect to offer is a DNA diagnostic test to determine a patient's ability to produce the enzyme essential for normal metabolism of drugs given to children with leukemia. We expect to subsequently expand our clinical testing services both by continuing to in-license tests as well as offering diagnostic tests that we discover and develop.
Database Services
We are creating a database that we call DNA BASE that contains the genomic position of gene variants, their frequency among various populations and their association with human traits, including disease. We believe the resultant annotated genome will be useful to pharmaceutical, diagnostic and managed care companies. We intend to non-exclusively license access to the aggregated data, while protecting the confidentiality of any individual data in the database.
DNA.com and the Gene Trust Project
We have launched our DNA.com website with the goal of recruiting volunteers into our genetic studies and becoming the leading source of genetic information and services on the Internet for physicians and consumers. We use patient populations developed through our Gene Trust project to validate our discoveries of gene variants identified through affected kinship or family-based linkage studies. DNA.com invites individuals to volunteer for the Gene Trust project. Those interested in participating in the Gene Trust project are asked to complete a profile that includes their own health information, medical histories and family disease histories. Individuals can update their information on an ongoing basis. This information, which is assigned an anonymous code number, is then analyzed by DNA Sciences' scientists for relevance to ongoing genetics research projects. Depending upon the result of that analysis, some individuals are offered the opportunity to participate in a specific study requiring them to give a blood sample containing DNA.
Over time, we expect to develop a large, active database containing this information and the genetic information we derive from the DNA samples. While academic and commercial researchers will be able to access our database of aggregated genetic information, no information about individual volunteers is ever made available to outside researchers without the consent of the study participant. We employ state-of-the art security protocols and technology, certified by third parties, to ensure privacy and confidentiality of all participants in our Gene Trust project. We retain full ownership of all data collected and generated through our Gene Trust project.
We have entered into an agreement with WebMD to maximize traffic to our site. For more information on this agreement, See "—Strategic Relationships" and "Related Party Transactions."
42
Technology and Development Platform
We have built a fully integrated DNA analysis facility that supports our business strategy and approach for the discovery of disease-associated gene variants.
Production Genotyping
We anticipate that it will be necessary to perform approximately two million STR and SNP genotypes to identify the gene variants associated with each common disease. To meet these genotyping requirements, we have built a production genotyping facility to perform high throughput genetic analysis using 48 Molecular Dynamics MegaBACE devices. We currently have the capacity to genotype approximately 40,000 SNPs or 50,000 STRs per day. We purchase supplies from established vendors, including Amersham Pharmacia Biotech and Applied Biosystems, to perform SNP and STR genotyping and DNA sequencing. We will continue to review the field of DNA analysis for lower cost sequencing and genotyping methodologies.
Production Process Management
Our informatics team has built a networked environment permitting the transfer, storage and retrieval of data from all of our operations in a web-based format to support our sequencing and genotyping management system. The database is secure, scalable, and some data sets can be viewed remotely by customers and collaborators. A major feature of this information system is a multi-terabyte Oracle 8i database.
Our production process management platform has two components: our production management system and our software suite that supports our genetic study design and analysis.
Production Management System
Our production management system is a web-enabled application that keeps track of all samples in an anonymous format, controls laboratory processes to eliminate sample switching, contamination or misuse of samples and manages inventories of reagents including primers and enzymes. We are currently developing an enhanced version of this system that can import data from external proprietary databases.
Software Support for Genetic Study Design and Analysis
A second software application called DNA Connects will permit physicians and scientists to:
43
Strategic Relationships
Clinical Research Agreement with the University of Utah
We entered into a research agreement with the University of Utah in December 2000, under which we plan to collaborate with researchers at the University to discover gene variants which contribute to common disease. Working with us, these researchers will access the Utah Population Database to identify potential participants for our genetic studies. Under our agreement, we may direct the University to perform database searches and related research under task orders for a given disease. The first disease we are studying under this agreement is cancer. We may also direct the University to perform database searches and related research for genetic studies of other diseases under additional task orders including diabetes, hypertension, cardiovascular disease and multiple sclerosis.
The University will obtain tissue samples from individuals who are identified by a database search and who agree to participate in our genetic studies, and will provide portions of the samples to us for our analysis of genetic variants associated with the relevant disease. While the University owns these samples, it agreed to grant us an exclusive right for five years to use the tissue samples to perform commercial research relating to the disease that is the subject of the task order. In addition, we have an option to obtain an exclusive license to commercialize products using, based upon or incorporating inventions made by the University or jointly by the University and us in the performance of the research agreements for therapeutic, diagnostic and pharmacogenomic products and services in the disease that is the subject of the task order. We also have a right of first negotiation to obtain an exclusive license to any other inventions made by University researchers through use of the tissue samples outside the scope of the collaboration during the five year period following our receipt of the samples.
Under this agreement, we must pay to the University an annual license fee for use of the tissues, and milestone payments and royalties on sales of products developed using tissue samples acquired from the University. If we exercise our option to obtain a license under the University's inventions made in the course of the collaboration, we must make milestones payments and pay royalties on sales of products covered by patents and patent applications owned by the University. Our access to the database or any collaborative efforts with University researchers can be terminated by the University upon 30 days notice.
Research, Development and Commercialization Agreement with Amersham Pharmacia Biotech Inc.
We entered into an agreement with Amersham Pharmacia Biotech Inc. in December 2000 under which we are collaborating with Amersham to develop and commercialize a microchannel DNA sequencer for use in performing DNA sequencing and genotyping studies. The agreement provides that the parties will establish a joint research and development team to develop the DNA sequencer pursuant to an agreed-upon plan. Amersham is required to fund this joint research and development effort and make cash payments to us totaling $7.5 million over a three year period. Under the agreement, we grant to Amersham a worldwide, royalty free license to our technology and intellectual property which the DNA sequencer utilizes to manufacture, distribute and sell the sequencer. Amersham has the exclusive right to manufacture, distribute and sell the microchannel DNA sequencer. We will receive a royalty on sales of the DNA sequencer.
In addition, under the agreement, Amersham must sell to us reagents at predetermined reduced prices for use in any DNA sequencing and genotyping which is outside the scope of our development of the DNA sequencer. We also have the right to purchase from Amersham some prototype and initial commercial versions of the device developed with our technology.
44
Collaboration Agreement with WebMD Corporation
To maximize traffic to DNA.com, we have partnered with WebMD. WebMD will promote our consumer-oriented genetics channel called The Family Genetics Channel, and our professional-oriented genetics channel. Additionally, by posting a link from the WebMD site to DNA.com, we will solicit participants on the consumer channel who are willing to donate blood samples to us for our genetic studies. We believe this partnering strategy takes advantage of a large and growing installed base of well-characterized patients. We believe this group of patients is more likely to participate as volunteers in the DNA Sciences Gene Trust project than those reached through broad mass marketing efforts. As a result, we believe this partnering strategy also reduces the need for expensive marketing to drive user traffic to DNA.com.
Under the agreement, we have the exclusive right to provide genetic and genomic information for these channels, although WebMD may post genetics and genomics content on these channels that it generates or that is provided for free by non-profit, academic or charitable sources, to the extent we are unable to provide such content. WebMD may include content related to genetics and genomics on its website outside of the genetics channels, but WebMD may not include content from our competitors for the purpose of advancing the business of our competitors. WebMD may not create or establish a channel on its website that is duplicative of our channels. This exclusivity does not apply to versions of WebMD that are modified for use in countries outside of the United States. For additional information relating to our agreement with WebMD, see "Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Related Party Transactions."
Intellectual Property
We do not currently own any patents. We own 19 application patent families and have licensed several additional patent filings from third parties. We have a reagent supply agreement with Amersham Pharmacia, which is a licensed supplier of reagents under patents owned by Orchid Biosciences and California Institute of Technology, among others.
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary rights are covered by valid and enforceable patents or are effectively maintained as trade secrets. We intend to try to protect our proprietary position by filing United States and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. The patent position of genomics and genetics companies involves complex legal and factual questions and, therefore, enforceability cannot be predicted with certainty. Patents, if issued, may be challenged, invalidated or circumvented. Thus, any patents that we own or license from third parties may not provide any protection against competitors. Our pending patent applications, those we may file in the future, or those we may license from third parties, may not result in patents being issued. Also, patent rights may not provide us with adequate proprietary protection or competitive advantages against competitors with similar technology. The laws of certain foreign countries do not protect our intellectual property rights to the same extent as do the laws of the United States.
In addition to patents, we rely on trade secrets and proprietary know-how. We seek protection, in part, through confidentiality and proprietary information agreements. These agreements may not provide meaningful protection for our technology or adequate remedies in the event of unauthorized use or disclosure of such information. The parties to these agreements may breach them. Also, our trade secrets may otherwise become known to, or be independently developed by, our competitors. Furthermore, others may independently develop similar technologies or duplicate any technology that we have developed.
Research has been conducted for many years in the genomics field. This has resulted in a substantial number of issued patents and an even larger number of still-pending patent applications.
45
Patent applications in the United States are, in most cases, maintained in secrecy until patents issue. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made. Our commercial success depends significantly on our ability to operate without infringing the patents and other proprietary rights of third parties. Our technologies may unintentionally infringe the patents or violate other proprietary rights of third parties. In the event of infringement or violation, we may be prevented from commercializating our products or services.
The biotechnology and pharmaceutical industries have been characterized by extensive litigation regarding patents and other intellectual property rights. The defense and prosecution of intellectual property suits, United States Patent and Trademark Office interference proceedings and related legal and administrative proceedings in the United States and internationally involve complex legal and factual questions. As a result, such proceedings are costly and time-consuming to pursue and their outcome is uncertain. Litigation may be necessary to enforce patents that we own or license; protect trade secrets or know-how that we own or license; or determine the enforceability, scope and validity of the proprietary rights of others.
If we become involved in any litigation, interference or other administrative proceedings, we will incur substantial expense and the efforts of our technical and management personnel will be significantly diverted. An adverse determination may subject us to loss of our proprietary position or to significant liabilities, or require us to seek licenses that may not be available from third parties. We may be restricted or prevented from selling our products, if any, in the event of an adverse determination in a judicial or administrative proceeding or if we fail to obtain necessary licenses. Costs associated with such arrangements may be substantial and may include ongoing royalties. Furthermore, we may not be able to obtain the necessary licenses on satisfactory terms, if at all. These outcomes will materially adversely affect our business, financial condition and results of operations.
Competition
We face, and will continue to face, intense competition in our gene discovery programs from organizations such as specialized biotechnology firms, pharmaceutical companies, universities and other research institutions, the Human Genome Project and other government-sponsored entities. A number of entities, including Celera Genomics and InCyte Genomics, Inc., are attempting to rapidly identify and patent genes and SNPs. Other companies, such as deCODE genetics, Inc. and Myriad Genetics, Inc., are attempting to identify gene variants responsible for causing disease for themselves or their pharmaceutical partners. In addition, genotyping technology companies, such as Affymetrix Inc., may enter the gene discovery business. Competition is also intense in the genomics database area. Companies such as InCyte Genomics and Celera Genomics could direct their efforts to developing databases containing clinical and genetic information. Competition is also formidable among the diagnostic companies, including Abbott Laboratories, Bayer AG, Roche Molecular Systems and Dade Behring Inc., which develop and market many diagnostic products. In addition, PPGx faces competition in providing genotyping services from universities, laboratory service and biotechnology companies, including Genaissance Pharmaceuticals, Inc. and Variagenics, Inc.
Many of our competitors, either alone or together with their collaborative partners, have substantially greater financial resources and larger research and development staffs than we do. These competitors may discover, characterize genes or gene variants that are associated with disease more rapidly than we can. In addition, they may obtain patents on their genetic discoveries or products or services based on those discoveries. Patents or other intellectual property rights held by others could limit our rights to use our technologies or commercialize diagnostic or therapeutic products or services.
46
In addition, we face, and will continue to face, intense competition from other companies for collaborative arrangements with pharmaceutical and biotechnology companies, for establishing relationships with academic institutions and for licenses to proprietary technology.
Government Regulation
Our business is regulated by a number of federal, state, local and foreign governmental entities. These governmental entities may enact laws that limit our operations. Furthermore, regulation by governmental authorities in the United States and other countries will be a significant factor in our ongoing research and development activities, as well as in the production and marketing of any products that may be developed by us or our licensees. Various federal statutes and regulations also govern or influence the testing, manufacturing, safety, labeling, storage, recordkeeping, and marketing of diagnostic and therapeutic products. The process of obtaining necessary approvals, and the subsequent compliance with applicable statutes and regulations, require the expenditure of substantial time and financial resources. Any failure by us or our collaborators, licensors or licensees to obtain, or any delay in obtaining, regulatory approval could have a material adverse effect on our business.
Laboratory Certification and Regulation
Through facilities acquired from PPGx, we operate a laboratory facility in North Carolina registered under CLIA. CLIA extends federal oversight to virtually all clinical laboratories by requiring that they be certified by the federal government or by a federally-approved accreditation agency. Pursuant to CLIA, clinical laboratories must meet quality assurance, quality control and personnel standards. Laboratories also must undergo proficiency testing and are subject to inspections. Standards for testing under CLIA are based on the complexity of the diagnostic tests performed by the laboratory, with all tests classified as either (1) high complexity, (2) moderate complexity, or (3) waived. Laboratories categorized as high complexity are required to meet more stringent requirements than moderate complexity laboratories. Laboratories performing only waived tests, which are tests determined to have a low potential for error and requiring little or no oversight, may apply for a certificate of waiver indicating that they need not comply with most of the requirements of CLIA. The sanction for failure to comply with CLIA requirements may be suspension, revocation or limitation of a laboratory's CLIA certificate, which is necessary to conduct business, as well as significant fines and/or criminal penalties. Our laboratory facility in North Carolina holds a current CLIA certificate that is appropriate for high complexity testing performed at the facility, and no deficiencies were noted during the laboratory's most recent CLIA inspection.
CLIA provides that a state may adopt regulations different from or more stringent than those under federal law, and a number of states do have their own laboratory regulatory schemes. State laws may require that laboratory personnel meet certain qualifications, specify certain quality controls, or require maintenance of certain records. North Carolina, where our laboratory facility is located, does not currently require laboratory licensure. However, some states, such as New York, require that a laboratory which is located outside of that state, but which accepts samples from the state, must be licensed, registered or otherwise authorized by the state. Furthermore, some states permit only certain authorized personnel, such as physicians or other licensed healthcare professionals, to order laboratory tests or receive laboratory test results. We have applied to the State of New York for the appropriate laboratory permit to do business and do not currently accept tissue samples for testing from New York residents. Furthermore, we are now evaluating the state laboratory licensure requirements in certain other states in order to conduct business in those states. However, we cannot be certain that we have obtained all necessary state authorizations in states where we currently do business.
47
Food and Drug Administration Regulation
Home Brew/Laboratory Developed Tests
We are currently developing diagnostic tests within our CLIA certified laboratory. We believe that these tests are likely to require continued certification of the laboratory as capable of performing high complexity tests. Currently, FDA does not regulate diagnostic tests developed in CLIA laboratories certified to conduct high complexity testing. Although FDA views such tests to be medical devices subject to the agency's authority, FDA has exercised its enforcement discretion to not require laboratories developing such tests, referred to as "home brew" or "in-house developed" tests, to seek approval or clearance of those tests at the present time. FDA has developed its home brew policy to permit laboratories meeting certain, specific operating criteria to continue to operate. These criteria include: (1) CLIA licensure, (2) CLIA certification as a laboratory capable of conducting high complexity tests, and (3) notification to physicians ordering such tests that the scientific validity of the test has not been reviewed by FDA. There can be no assurance that FDA will continue to refrain from exercising jurisdiction over such tests. If FDA chooses to regulate such tests, we could be required to obtain FDA approval to market our home brew tests. We cannot be certain that this regulatory approval would be obtained.
Overview of Device Regulation
Each medical device that we wish to commercially distribute in the U.S. will likely require either 510(k) clearance or PMA approval prior to marketing from the FDA under the Federal Food, Drug, and Cosmetic Act. Devices deemed to pose relatively less risk are placed in either class I or II, which require the manufacturer to submit a premarket notification requesting permission for commercial distribution, known as 510(k) clearance. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously 510(k) cleared device, are placed in Class III requiring PMA approval. We believe that most, if not all, of our diagnostic tests kits will be Class III devices that require a PMA.
The PMA approval process requires proof of the safety and effectiveness of the device to the FDA's satisfaction. A PMA application for a particular device must provide extensive preclinical and clinical trial data regarding the device and information regarding its design, manufacturing and labeling. After approval of a PMA, a new PMA or PMA supplement is required in the event of a modification to the device, its labeling or its manufacturing process. The PMA approval process is much more costly, lengthy and uncertain than a 510(k) notification. It generally takes from one to three years or even longer. A clinical trial is generally required to support a PMA application.
After a device is placed on the market, numerous regulatory requirements apply. These include: (1) the Quality System Regulation, which requires manufacturers to follow elaborate design, testing, control, documentation and other quality assurance procedures during the manufacturing process; (2) labeling regulations; (3) the FDA's general prohibition against promoting products for unapproved or "off-label" uses; and (4) the Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. Noncompliance with applicable requirements can result in failure of the government to grant premarket clearance or approval for devices, withdrawal of clearances or approvals, total or partial suspension of production, fines, injunctions, civil penalties, recall or seizure of products, and criminal prosecution.
Overview of Therapeutic Products
The FDA regulates therapeutic products either as drugs or as biologics. Regulations governing both drugs and biologics require the development and submission of substantial data establishing the
48
safety and efficiency of such products as well as information regarding the physical/chemical make-up of the product, manufacturing process and facility. The FDA must review and approve such submissions prior to marketing of either drugs or biological products. Once marketed, manufacturers of such products have numerous continuing regulatory obligations including those related to safety reports, manufacturing, advertising and promotion. Violations of these regulatory obligations can result in denial of product approvals, warning letters, recalls, product seizure, injunctions or criminal prosecutions.
Confidentiality of Health Information
Federal and state laws regulating the confidentiality and privacy of patient health information apply to us and our institutional collaborators who furnish us with DNA samples. Violations of these laws may result in the imposition of civil and/or criminal penalties, as well as actions for damages. Federal and state laws aimed at protecting the privacy of confidential patient health information vary widely, and the application of these laws in the context of genotyping services is evolving. These laws may affect our ability, as well as the ability of our institutional collaborators, to obtain and transmit genetic information. For example, we may be subject to penalties or regulatory action under these laws in the event that confidential information about any participant in our Gene Trust project is inadvertently disclosed to outside individuals or entities without the participant's consent or authorization. Likewise, we and our collaborators could be subject to such penalties or regulatory actions if our collaborators have not obtained appropriate authorizations prior to providing us with DNA samples.
The Health Insurance Portability and Accountability Act of 1996, or HIPAA includes provisions that affect how health information is used and disclosed. Pursuant to HIPAA, on December 28, 2000, the Department of Health and Human Services published the final regulations concerning the privacy of patient health information. The core requirements of the HIPAA rule generally apply to individually identifiable health information. The HIPAA rule requires, among other things, that health plans, healthcare providers or healthcare data clearinghouses, generally obtain specific consent or authorization of the patient for uses and disclosures of protected health information. The HIPAA rule also requires covered entities to provide notice to the patient regarding the uses and disclosures of protected health information and the covered entities' legal duties with respect to protected health information. With respect to research related activities, the HIPAA rule requires covered entities to either obtain a patient authorization for research related to treatment or a waiver of authorization from an IRB or a privacy board. For purposes of the civil and criminal penalties established in the statute, covered entities are not required to come into compliance with the requirements of HIPAA until February 2003.
Our services providing diagnostic testing at the request of healthcare professionals make us a covered entity for purposes of complying with the HIPAA privacy regulations. We expect to comply with the requirements of the regulations in our transactions with these professionals. We have no plans to use patient samples sent to us for diagnostic purposes in our research. With respect to the samples and information furnished to us by academic medical centers and others, we expect to comply with the regulation by continuing to ensure that an IRB approves our use of the samples and other information.
In addition to the HIPAA provisions described above, there are a number of state laws regarding the confidentiality of medical information which may continue to apply to our operations. State laws aimed at protecting the privacy of confidential patient health information, including genetic information, are many and varied, and states frequently adopt new laws in this area. Many state health information privacy laws apply only to specified healthcare providers or payers. Some state health information privacy laws commonly restrict the use and disclosure of medical information without patient consent or absent some type of IRB approval of a research protocol involving the information. Some states have laws specifically addressing genetic discrimination and genetic privacy. These laws
49
may, among other things, set standards for informed consent for obtaining genetic information and provide requirements related to the retention, disclosure, and destruction of genetic information.
The confidentiality of health information is a high priority for us, and we have policies and procedures in place to protect against unauthorized access to individually identifiable health information and to ensure that such information is handled appropriately. We expect that the institutional collaborators who furnish us DNA samples will do so under IRB-approved research and study protocols. Although our collaborators at the University of Utah have applied for IRB approval to collect DNA samples for use in our research, such approval has not yet been granted. Likewise, our study protocol for the Gene Trust project has received IRB approval, although some third parties have publicly raised concerns regarding the informed consent procedures we follow in recruiting volunteers through the Gene Trust project. We have designed our informed consent protocol to meet the requirements of applicable state genetic testing and informed consent laws, and it is our policy that no information about individual participants in the Gene Trust project is made available to outside researchers without the consent of the participant. We employ state-of-the-art security procedures and technology to ensure the confidentiality of information submitted to us by participants. Furthermore, we remove patient-identifying information before providing aggregated genetic information to academic and commercial researchers. Although many state health information privacy laws do not apply to "aggregate," "anonymized" or "de-identified" data, definitions of whether data has been anonymized vary and we cannot provide assurances that the data we currently furnish to our customers would be considered "anonymous" or "de-identified" under all laws. Notwithstanding our efforts in this area, we cannot provide assurances that our current ways of doing business substantially comply with applicable laws concerning the confidentiality of medical information, including genetic information, and that our current activities would not be the subject of enforcement proceedings or lead to liability under currently applicable health information privacy laws.
Reimbursement
Presently, PPGx markets to physicians a diagnostic test for childhood leukemia. Physicians administering the test may claim reimbursement for the test from patients and third party payers. We do not currently submit reimbursement claims to patients or third party payers for any diagnostic and therapeutic products and services. Our ability to successfully commercialize diagnostic and therapeutic products in the future may depend on the extent to which such products are reimbursed by third party payers, including governmental payers such as Medicare and Medicaid, and private health insurers. These third-party payers are increasingly likely to challenge the prices charged for healthcare products and services. Government and other third party payers are increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for new products. These and other factors may result in lower prices for healthcare products and services commercialized by us, our customers and our collaborative partners.
Employees
We had 97 full-time employees as of December 29, 2000, 18 of whom hold Ph.D. or M.D. degrees, or both, and 15 of whom hold other advanced degrees. Of our total workforce, 66 are engaged in research and development activities, 31 are engaged in business development, finance and administration. PPGx has 49 full time employees located primarily in their facilities in Research Triangle, North Carolina and La Jolla, California. They also employ three persons in their certified laboratory in Cambridge, England. None of our or PPGx's employees are represented by a collective bargaining agreement. We believe that relations with our and PPGx's employees are good.
50
Facilities
We currently lease a 63,924 square foot facility in Fremont, California, which expires in September 2007. We occupy 6,000 square feet of Amersham Biotech Pharmacia laboratory space in Sunnyvale, California. We are in the process of relocating our Sunnyvale operation to the Fremont facility. We expect this process to be complete by February 2001.
PPGx leases 7,437 square feet of office and laboratory space in Research Triangle Park, North Carolina, 14,091 square feet of lab and office space in La Jolla, California, 1,344 square feet of lab and office space in Cambridge, England, 662 square feet of lab and office space in Cambridge, Massachusetts and 352 square feet of lab and office space in Philadelphia, Pennsylvania. We believe that our facilities are adequate for our and PPGx's needs for the foreseeable future.
Legal Proceedings
We are currently not a party to any material legal proceedings.
51
Executive Officers, Directors and Key Personnel
The following table sets forth information regarding our executive officers, directors and key personnel as of December 29, 2000.
| Name |
Age |
Position |
||
|---|---|---|---|---|
| Executive Officers and Directors | ||||
| Hugh Y. Rienhoff, Jr., M.D. | 48 | Chairman and Chief Executive Officer | ||
| Gregory T. Went, Ph.D. | 37 | Chief Operating Officer and Director | ||
| Susan Berland | 46 | Chief Financial Officer | ||
| Steven B. Lehrer | 41 | Chief Business Officer | ||
| Ray L. White, Ph.D. | 55 | Chief Scientific Officer | ||
| Joshua S. Baker, Ph.D. | 45 | Chief Executive Officer of PPGx, Inc. | ||
| Brian Atwood | 49 | Director | ||
| Walter Burlock | 38 | Director | ||
| James Clark, Ph.D. | 54 | Director | ||
| James D. Watson, Ph.D. | 70 | Director | ||
Key Personnel |
||||
| Richard D. Dvorak | 40 | Vice President, Operations | ||
| Tom Kent | 42 | Vice President, Software Engineering | ||
| Ted Richards | 49 | Executive Creative Director | ||
| Ezra Van Gelder, M.S. | 49 | Vice President, Technology Development |
Hugh Y. Rienhoff, Jr., M.D., founded DNA Sciences in 1998 and has served as Chief Executive Officer and Chairman of the Board since our inception. From March 1997 to April 1998, Dr. Rienhoff served as a director of Abingworth Management Ltd., a venture capital firm. From July 1992 to February 1997, he was a partner at New Enterprise Associates, a venture investment firm. Dr. Rienhoff was a founding director of Healtheon Corporation, a health related Internet company, now known as WebMD and is currently a member of the board of directors of Aurora Bioscience, Inc., a biotechnology company, and Microcide Pharmaceuticals, Inc., a biopharmaceutical company. Dr. Rienhoff received his M.D. from The Johns Hopkins University School of Medicine and his B.A. from Williams College.
Gregory T. Went, Ph.D., has served as our Chief Operating Officer and as a director since January 2000. In 1993, Dr. Went co-founded CuraGen Corporation, a biotechnology corporation, where he served as a director and Executive Vice President until January 1999. From February 1999 to January 2000, Dr. Went pursued personal investments. Dr. Went received his Ph.D. in Chemical Engineering from the University of California at Berkeley and his B.S. in Chemical Engineering and Informational Sciences from Carnegie Mellon University.
Susan Berland has served as our Chief Financial Officer since September 2000. From September 1999 to August 2000, she served as head of financial planning of Monsanto Company, a life sciences company. From April 1996 to August 1999, she served as Director-Mergers & Acquisitions, where she was involved in strategic business transactions. From June 1994 to March 1996, Ms. Berland was Senior Manager-Treasury for Holnam Inc, a manufacturing company. From June 1980 to June 1994, Ms. Berland served in a variety of corporate finance and strategic planning roles for Ameritech Corporation, a telecommunications company. Ms. Berland received her M.B.A. and her B.A. in Business Administration from the University of Wisconsin.
Steven B. Lehrer has served as our Chief Business Officer since February 2000. From January 1999 to January 2000, Mr. Lehrer served as co-leader of the Specialty Crop Strategic Business Team and President of Integrated Protein Technologies at Monsanto Company. From January 1995 to April 1999,
52
Mr. Lehrer started and led the Pharmaceutical Technology Business Team and helped create Monsanto Health Solutions, an operating unit of Monsanto Company. From 1992 to 1995, Mr. Lehrer held various general management, business development and planning positions with Monsanto. He received his M.B.A. from Harvard Business School, and his B.S. in Chemical Engineering and B.A. in Economics from the University of Maryland.
Ray L. White, Ph.D., has served as our Chief Scientific Officer since July 2000. Dr. White founded the Huntsman Cancer Institute and served as an Executive Director from 1994 to September 1999. Dr. White served as Professor and Chairman of the Department of Oncological Sciences at the University of Utah from 1994 to July 2000. Dr. White has been a professor of Human Genetics and has served as the Co-Chairman of the Department of Human Genetics at the University of Utah from 1984 to 1994. Dr. White received his Ph.D. in Microbiology from the Massachusetts Institute of Technology and his B.S. in General Science from the University of Oregon.
Joshua S. Baker, Ph.D., has served as the Chief Executive Officer of our subsidiary, PPGx, since December 2000. From October 1999 to December 2000, he served as President and Chief Executive Officer of PPGx. From September 1997 to October 1999, Dr. Baker served as Executive Vice President of Global Operations for PPD, Inc., a contract research services company. From May 1994 to September 1997, Dr. Baker held various management positions with PPD, Inc. He received his Ph.D. in Statistics from Texas A&M University and his B.S. in Mathematical Sciences from Penn State University.
Brian Atwood has served as a director since March 1999. Mr. Atwood is a managing director at Versant Ventures, a venture capital firm. From November 1995 to September 1997, Mr. Atwood was a venture partner at Brentwood Venture Capital, and has been a general partner of Brentwood Venture Capital since October 1997. From 1993 to 1995, Mr. Atwood served as President and Chief Executive Officer of Glycomed Inc., a biotechnology company subsequently acquired by Ligand Pharmaceuticals, Inc. From 1988 to 1993, Mr. Atwood also served as Vice President of Operations of Glycomed. From 1986 to 1987, Mr. Atwood was a director at Perkin-Elmer/Cetus Instrument Systems, a joint venture formed by Perkin-Elmer Corp. and Cetus Corporation. Mr. Atwood received his M.B.A. from Harvard Business School, his M.S. in Ecology from the University of California, Davis and his B.S. in Biology from the University of California, Irvine.
Walter Burlock has served as a director since March 2000. From August 1990 to July 2000, Mr. Burlock served as a managing director of Soros Fund Management LLC, a global investment management company. From January 1996 to December 1996, Mr. Burlock was on sabbatical from Soros. Since July 2000, Mr. Burlock has served as managing member at Origin Capital Management LLC and he continues to serve as a consultant to the Soros Funds. He also serves on the board of directors of Centene Corp., a privately held managed care company. Mr. Burlock received his B.S. in Physics and Mathematics from Bowdoin College.
James Clark, Ph.D., has served as a director since our inception. Dr. Clark founded and served as Chairman of the board of directors of Healtheon Corporation, a health related Internet company that merged with WebMD, from its inception in December 1995 until October 2000. Dr. Clark co-founded Netscape Communications Corporation, an Internet browser company, in 1994 and has served as the Chairman of the board of directors of Netscape from its inception until March 1999. He also served as President and Chief Executive Officer of Netscape from its inception until 1994. From 1981 until 1994, Dr. Clark served as Chairman of the board of directors of Silicon Graphics, Inc., a computer company that he founded in 1981. Prior to founding Silicon Graphics, Dr. Clark was an Associate Professor at Stanford University. He received his Ph.D. in Computer Science from the University of Utah and his M.S. and B.S. in Physics from the University of New Orleans.
James D. Watson, Ph.D., has served as a director since April 2000. Since 1994, Dr. Watson has served as President of the Cold Spring Harbor Laboratories, a research and educational institution.
53
From 1988 to 1992, Dr. Watson served as a director of the Human Genome Project. Dr. Watson and Dr. Francis Crick were awarded the Nobel Prize in Medicine in 1962 for successfully proposing the double helical structure of DNA. Dr. Watson received his Ph.D. in Genetics from Indiana University and his B.S. in Zoology from the University of Chicago.
Richard D. Dvorak has served as our Vice President of Operations since January 2000 and has served as our Director of Operations from September 1999 to January 2000. Mr. Dvorak has served in various capacities at RayChem Corporation, a chemical and materials manufacturing company, from April 1996 to July 1999. From July 1997 to July 1999, Mr. Dvorak served as a Director of Electronic North American Supply Chain at RayChem Corporation, managing the Americas/Pacific Rim Inside Sales, product support groups and Master Data Groups. From April 1996 to July 1997, Mr. Dvorak was an Electronics Inside Sales Manager and from 1989 to April 1996, he was a Logistics Manager. Mr. Dvorak received his M.B.A. from Dartmouth College and his B.S. in Chemical Engineering from Stanford University.
Tom Kent has served as our Vice President of Software Engineering since August 2000. During the ten years prior to August 2000, Mr. Kent worked as an independent consultant providing strategic and tactical software engineering consulting for numerous companies, including IBM, Hewlett-Packard, and Intel. During this time, Mr. Kent also served as President of Active Software Corporation, f/k/a ServiceLink Systems, a software technology company which he founded. Mr. Kent received his M.B.A. from Santa Clara University and his B.S. in Computer Science from the University of Utah.
Ted Richards has served as our Executive Creative Director since July 2000. From July 1996 to July 2000, Mr. Richards served as Vice President and Creative Director at AudioHighway.com, an Internet company. From 1994 to July 1996, Mr. Richards served as Vice President of Interactive Design for The SoftAd Group. Mr. Richards received his B.A. in Creative Writing and Philosophy from San Francisco State University.
Ezra Van Gelder, M.S., has served as our Vice President of Technology Development since February 1999. From September 1996 to February 1999, Mr.Van Gelder was a senior engineering manager for KLA/Tencor Corporation. From August 1995 to September 1996, he was Director of Engineering at Alara Inc. Prior to that time, he served as an engineering program manager at Molecular Dynamics, Inc., now a part of Amersham Pharmacia Biotech. Mr. Van Gelder received his M.S. in Mechanical Engineering from Santa Clara Univesity and his B.S. in Mechanical Engineering from the University of Tel Aviv.
Board Committees
Audit Committee. Our audit committee currently consists of Messrs. Atwood and Burlock and Dr. Clark. The audit committee reviews our internal accounting procedures and the results and scope of our annual audit and the services provided by our independent auditors.
Compensation Committee. Our compensation committee currently consists of Dr. Clark and Mr. Burlock. The compensation committee administers our stock option plans, reviews and approves the compensation and benefits of all our officers and establishes and reviews general policies relating to compensation and benefits of our employees.
Compensation Committee Interlocks and Insider Participation
None of our executive officers serves as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our board of directors or compensation committee.
54
Board Composition
We currently have six directors. Upon the closing of this offering, the terms of the office of the board of directors will be divided into three classes. As a result, a portion of our board of directors will be elected each year. The division of the three classes, the initial directors and their respective election dates are as follows:
At each annual meeting of stockholders after the initial classification, the successors to directors whose terms will then expire will be elected to serve from the time of election and qualification until the third annual meeting following election. In addition, the authorized number of directors may be changed only by resolution of the board of directors. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. This classification of the board of directors may have the effect of delaying or preventing changes in control or management.
Director Compensation
With the exception of Dr. Watson, our directors do not receive cash compensation for services on our board of directors or any board committee. Dr. Watson receives $2,500 for each board meeting he attends.
In July 2000, we granted Dr. Watson an option to purchase 32,000 shares of common stock at an exercise price of $0.75 per share. In September 2000, we also granted Dr. Watson an option to purchase 32,000 shares of common stock at an exercise price of $0.75 per share in consideration for services to be performed as a member of our Scientific Advisory Board. These options vest monthly in equal installments over forty-eight months.
Our 2001 Non-Employee Directors' Stock Option Plan provides for the automatic grant of options to purchase shares of common stock to our non-employee directors. Any non-employee director elected after the close of this offering will be granted an initial option to purchase 16,000 shares of common stock. Starting at the annual stockholder meeting in 2002, each of our non-employee directors who has served as a non-employee director for at least six months will automatically receive an option to purchase 4,000 shares of common stock. See "Benefit Plans—2001 Non-Employee Directors' Stock Option Plan" below for a more detailed explanation of the terms of these stock options.
55
Executive Compensation
The following table summarizes the compensation earned for services rendered to us for the fiscal year ended December 31, 2000 by our chief executive officer and our three most highly compensated executive officers whose compensation exceeded $100,000 during the fiscal year ended December 31, 2000. These executives are referred to as the named executive officers elsewhere in this prospectus.
| |
|
Long-Term Compensation |
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Name and Principal Position |
Annual Compensation Salary |
Shares Underlying Options |
All Other Compensation |
||||||
| Hugh Y. Rienhoff, Jr., M.D. Chairman and Chief Executive Officer |
$ | 273,951 | 530,000 | $ | 43,435 (1 | ) | |||
| Gregory T. Went, Ph.D. Chief Operating Officer |
$ | 238,958 | 50,000 | $ | 181,798 (2 | ) | |||
| Steven B. Lehrer Chief Business Officer |
$ | 216,451 | 375,000 | $ | 27,312 (3 | ) | |||
| Ray L. White Chief Scientific Officer |
$ | 125,000 | 850,000 | $ | 21,825 (4 | ) | |||
56
Option Grants
The following table sets forth certain information with respect to stock options granted to each of the named executive officers in the fiscal year ended December 31, 2000, including the potential realizable value over the ten-year term of the options, based on assumed rates of stock appreciation of 5% and 10%, compounded annually. These assumed rates of appreciation comply with the rules of the SEC and do not represent our estimate of future stock price. Actual gains, if any, on stock option exercises will be dependent on the future performance of our common stock. The assumed 5% and 10% rates of stock appreciation are based on an assumed initial public offering price of $ per share.
In the fiscal year ended December 31, 2000, we granted options to purchase up to an aggregate of 5,226,324 shares of common stock to employees, directors and consultants under our options plans and outside our option plans. In addition, we have reserved 305,727 shares for issuance upon exercise of stock options which we assumed in connection with our acquisition of PPGx. All options were issued at exercise prices at or above the fair market value of our common stock on the date of grant, as determined in good faith by the board of directors. In general, our options vest over five years with 20% of the total shares vesting after one year and the remaining shares vesting in equal monthly installments for the next 48 months. All options have a term of up to ten years.
Option Grants During Year Ended December 31, 2000
| |
|
|
|
|
Potential Realizable Value at Assumed Annual Rates of Stock Price Appreciate for Option Term |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Percentage of Total Options Granted to Employees in Fiscal 2000 |
|
|
|||||||||
| |
Number of Securities Underlying Options Granted |
|
|
||||||||||
| |
Exercise or Base Price per share |
|
|||||||||||
| |
Expiration Date |
||||||||||||
| Name |
5% |
10% |
|||||||||||
| Hugh Y. Rienhoff, Jr. | 250,000 | 4.8 | % | $ | 0.75 | 10/2/10 | |||||||
| 280,000 | 5.4 | 2.05 | 12/5/10 | ||||||||||
Gregory T. Went |
50,000 |
0.9 |
2.05 |
12/5/10 |
|||||||||
Steven B. Lehrer |
300,000 |
(1) |
5.7 |
0.75 |
10/2/08 |
||||||||
| 75,000 | 1.4 | 2.05 | 12/5/10 | ||||||||||
Ray L. White |
200,000 |
3.8 |
0.75 |
7/26/10 |
|||||||||
| 300,000 | 5.7 | 0.75 | 7/26/10 | ||||||||||
| 300,000 | (1) | 5.7 | 0.75 | 10/2/08 | |||||||||
| 50,000 | 0.9 | 2.05 | 12/5/10 | ||||||||||
57
Aggregated Option Exercises and Option Values
The following table sets forth summary information for our named executive officers, the shares acquired and the value realized on each exercise of stock options during fiscal year ended December 31, 2000, and the number and value of exercisable and unexercisable options held by them as of December 31, 2000. Amounts shown in the value realized column and the value of unexercised in-the-money options at December 31, 2000 column are based on an assumed initial public offering price of $ per share without taking into account any taxes that may be payable in connection with the transaction, multiplied by the number of shares underlying the option, less the aggregate exercise price payable for these shares.
Aggregated Option Exercises in 2000 and Fiscal Year-End Option Values
| Name |
Shares Acquired on Exercise |
Value Realized |
Number of Securities Underlying Unexercised Options at December 31, 2000 Exercisable/Unexercisable(1) |
Value of Unexercised In-the-Money Options at December 31, 2000 Exercisable/Unexercisable(1) |
||||
|---|---|---|---|---|---|---|---|---|
| Hugh Y. Rienhoff, Jr. | 250,000 | 0/280,000 | ||||||
| Gregory T. Went | — | 0/50,000 | ||||||
| Steven B. Lehrer | — | 300,000/75,000 | ||||||
| Ray L. White | 200,000 | 300,000/350,000 |
Benefit Plans
2001 Equity Incentive Plan
In January 2001 our board of directors adopted and in our stockholders approved our 2001 Equity Incentive Plan, which amends and restates our 2000 Equity Incentive Plan. The incentive plan will become effective on the closing date of this offering and initially authorizes 9,000,000 shares of our common stock for issuance. In addition, on each January 1, starting with January 1, 2002 and ending on January 1, 2010, the aggregate number of shares of common stock that may be issued under the incentive plan automatically will be increased by the lower of:
Shares subject to stock awards that have expired or otherwise terminated without having been exercised in full again become available for the grant of awards under the incentive plan.
Our incentive plan provides for the grant of incentive stock options, as defined in Section 422 of the Internal Revenue Code, and nonstatutory stock options, restricted stock purchase awards and stock bonuses. We may grant these awards to employees, officers, directors and consultants and those of our affiliates.
The incentive plan is administered by the board of directors or a committee appointed by our board of directors. The board or the committee determines recipients and types of awards to be granted, including the number of shares subject to an award, the vesting schedule of awards, the
58
exercisability of awards and, subject to applicable restrictions, other terms of awards. Our board of directors has delegated administration of the incentive plan to the compensation committee.
Our compensation committee determines the price of stock options granted under the incentive plan, provided that the exercise price for an incentive stock option cannot be less than 100% of the fair market value of our common stock on the date of grant. Options granted under the incentive plan vest at the rate determined by our compensation committee. Typically, options granted under the incentive plan vest over five years, with 20% vesting at the end of one year and the remainder vesting pro rata monthly thereafter, though options granted in the future may vest at other rates. The term of stock options granted under the incentive plan generally may not exceed 10 years. Vested options may, as a general rule, be exercised at any time.
Generally, an optionholder may not transfer a stock option other than by will or the laws of descent and distribution unless the optionholder holds a nonstatutory stock option that provides otherwise. However, an optionholder may designate a beneficiary who may exercise the option following the optionholder's death. An optionholder whose service relationship with us or any of our affiliates ceases for any reason may exercise the option to the extent it was vested for the term provided in the stock option agreement. Options generally expire three months after the termination of an optionholder's service. However, if an optionholder is permanently disabled or dies during his or her service, that person's options generally may be exercised up to 12 months following disability or death.
The Internal Revenue Code sets forth other restrictions applicable to options that are intended to be incentive stock options, and awards made under this plan will comply with these restrictions.
If we become subject to Section 162(m) of the Internal Revenue Code no person may be granted stock options under the incentive plan covering more than 4,000,000 shares of our common stock in any calendar year. Section 162(m) denies a deduction to publicly held companies for certain compensation paid to specified employees in a taxable year to the extent the compensation exceeds $1,000,000. In addition, the stock plan is designed to comply with the conditions for exemption from the short-swing profit recovery rules under Rule 16b-3 under the Securities Exchange Act.
Restricted stock purchase awards granted under the incentive plan may be granted pursuant to a repurchase option in our favor that will lapse in accordance with a vesting schedule and at a price determined by the board of directors or a committee appointed by the board of directors. Stock bonuses may be awarded in consideration of past services without a purchase payment. Rights under a stock bonus or a restricted stock purchase award are transferable only upon such terms and conditions as are set forth in the relevant agreement, as determined by the board of directors or the committee appointed by the board of directors in its sole discretion.
In the event of certain corporate transactions including, but not limited to, a sale, lease or other disposition of all or substantially all of our assets, a merger or a consolidation, all outstanding awards under the incentive plan either will be assumed, continued or substituted by any surviving entity. If assumed, the awards will generally be subject to the same terms and conditions as were in effect before the transaction, including the vesting schedule. If the surviving entity decides not to assume, continue or substitute the outstanding awards, then with respect to stock awards held by persons providing us or any of our affiliates service, the vesting of the award and, if applicable, the time during which the award may be exercised will be accelerated in full. Stock awards will terminate if not exercised if applicable before the corporate transaction.
The board of directors may amend the incentive plan at any time. Amendments will be submitted for stockholder approval to the extent required by applicable law. The incentive plan will terminate on the day before the tenth year anniversary of the date of adoption of the incentive plan in amended and restated form, January 3, 2011, unless sooner terminated by the board of directors or a committee appointed by the board of directors.
59
As of December 29, 2000, there were options outstanding to purchase an aggregate of 3,617,637 shares of our common stock. As of December 29, 2000, stock bonuses and restricted stock awards covering 3,075,500 shares of our common stock have been granted and were outstanding under the incentive plan. As of December 29, 2000, 1,295,873 shares remained available for grant.
2001 Employee Stock Purchase Plan
In January 2001 our board of directors adopted and in our stockholders approved the 2001 Employee Stock Purchase Plan. The purchase plan covers an aggregate of 500,000 shares of our common stock. However, on each January 1, starting with January 1, 2002 and ending on January 1, 2011, the aggregate number of the shares of common stock that may be issued under the purchase plan automatically will be increased by the lesser of:
The purchase plan will become effective on the first day of trading of our common stock on the Nasdaq National Market. The purchase plan is intended to qualify as an "employee stock purchase plan" within the meaning of Section 423 of the Internal Revenue Code.
The purchase plan provides a means by which our employees and our designated affiliates may purchase our common stock through payroll deductions. The purchase plan is implemented by offerings of rights to eligible employees. Under the purchase plan, we may specify offerings with a duration of not more than 27 months, and may specify shorter purchase periods within each offering. The first offering will begin on the date the shares are first sold to the public in this offering and will end on January 31, 2003. Purchases during the first offering will occur on January 31, 2002, July 31, 2002 and January 31, 2003.
Unless otherwise determined by our board of directors, common stock is purchased for accounts of employees participating in the purchase plan at a price per share generally equal to the lower of (A) 85% of the fair market value of a share of common stock on the first day of the offering or (B) 85% of the fair market value of a share of common stock on the date of purchase. The board may provide that employees who become eligible to participate after the offering begins nevertheless may enroll in the offering. Generally, all regular employees, including executive officers, who work at least 20 hours per week, who are customarily employed by us or by any of our affiliates for at least five months per calendar year and who are employed by us or by one of our affiliates at the start of an offering, or at the start of a purchase period within an offering, may participate in the purchase plan. Participants may authorize payroll deductions of up to 15% of their base compensation for the purchase of stock under the purchase plan.
The fair market value of the shares on the first date of the first offering will be the price per share at which our shares are first sold to the public as specified in the final prospectus with respect to this offering. Otherwise, fair market value generally means the closing sales price, rounded up where necessary to the nearest whole cent, for such shares or the closing bid, if no sales were reported, as quoted on the Nasdaq National Market on the trading day prior to the relevant determination date, as reported in The Wall Street Journal.
Eligible employees may be granted rights only if the rights together with any other rights granted under employee stock purchase plans do not permit such employee's rights to purchase our common stock to accrue at a rate which exceeds $25,000 of fair market value of our common stock for each calendar year in which such rights are outstanding. Additionally, for the first offering, no employee may purchase more than 10,000 shares of common stock on any one purchase date. No employee will be
60
eligible for the grant of any rights under the purchase plan if immediately after such rights are granted, the employee has voting power over 5% or more of our outstanding capital stock.
In the event of certain specified types of corporate transactions including, but not limited to, certain specified types of merger, consolidation or other transactions, our board of directors has discretion to provide that each right to purchase common stock will be assumed or an equivalent right substituted by the successor corporation, or our board of directors may shorten the offering period and provide for all sums collected by payroll deductions to be applied to purchase stock immediately prior to the corporate transaction.
The purchase plan will terminate at the board's discretion or when all of the shares reserved for issuance have been purchased.
2001 Non-Employee Directors' Stock Option Plan
In January 2001 our board of directors adopted and in our stockholders approved the 2001 Non-Employee Directors' Stock Option Plan. The directors' plan provides for the automatic grant of nonstatutory stock options to our non-employee directors to purchase shares of our common stock. The directors' plan will become effective on the closing of this offering. Our board of directors administers the directors' plan, unless our board of directors delegates administration to a committee. The aggregate number of shares of common stock that may be issued pursuant to options granted under the directors' plan is 340,000 shares of common stock.
Pursuant to the terms of the directors' plan, each director who is elected on or after the effective date of this offering and is not otherwise an employee or consultant of DNA Sciences or any of our affiliates automatically will be granted an initial nonstatutory stock option to purchase 16,000 shares of our common stock upon his or her initial election or appointment to our board of directors. Commencing in 2002 at the annual meeting of our stockholders, each non-employee director automatically will receive an annual nonstatutory stock option for 4,000 shares of our common stock. For initial grants, one-quarter of the shares will vest on each of the first four anniversaries of the date of grant. For annual grants, one-half of the shares will vest six months from the date of grant and the remaining one-half will vest on the first anniversary of the date of grant. Vested options may, as a general rule, be exercised at any time.
The exercise price of the options granted under the directors' plan will be equal to the fair market value of our common stock on the date of grant. No option granted under the directors' plan may be exercised after the expiration of 10 years from the date it was granted. Options granted under the directors' plan generally are not transferable, unless provided otherwise in the stock option agreement. However, an optionholder may designate a beneficiary who may exercise the option following the optionholder's death. An optionholder whose service relationship with us or any of our affiliates, whether as a non-employee director or, subsequently, an employee, director or consultant of either us or any of our affiliates, ceases for any reason may exercise an option to the extent it is vested for the term provided in the stock option agreement. Options will expire three months after termination of the optionholder's service. However, if an optionholder is permanently disabled or dies during his or her service, that person's options may be exercised up to 12 months following disability of death.
In the event of certain changes in control in our ownership, options outstanding under the directors' plan will automatically become vested and will terminate if not exercised prior to such a change in control. The directors' plan is scheduled to terminate on the day prior to the tenth anniversary of the date of its adoption by our board of directors, unless sooner terminated by our board of directors.
61
PPGx, Inc. 1999 Equity Incentive Plan
We assumed the PPGx, Inc. 1999 Equity Incentive Plan in connection with our acquisition of PPGx. The PPGx plan will continue in existence; however no further stock awards will be granted under the plan. We assumed all outstanding stock awards under the PPGx plan and these stock awards were converted into stock awards to acquire shares of our common stock. With the exception of the exercise price and the number of shares covered by the stock awards which were converted pursuant to a formula set forth in the agreement for the acquisition of PPGx, all the terms of the stock awards granted under the PPGx plan and assumed by us have remained the same. As of December 29, 2000, there were options outstanding to purchase an aggregate of 305,727 shares of our common stock that we assumed as a result of the acquisition and that had been issued under the PPGx plan. As of December 29, 2000, no stock bonuses or restricted stock awards had been granted under the PPGx Plan.
Options granted under the PPGx plan vest at the rate determined by the PPGx Board or the committee and specified in the option grant notice or option agreement. Typically, options granted under the PPGx plan vest over four years with the first 12.5% of the shares covered by the option vesting on the six-month anniversary of the grant and the remaining 87.5% of the shares covered by the option vesting monthly thereafter.
401(k) Plan
We sponsor a defined contribution plan intended to qualify under Section 401(k) of the Internal Revenue Code. All employees are eligible to participate. Participants may make pre-tax contributions to the 401(k) plan of up to 15% of their eligible earnings, subject to a statutorily prescribed annual limit ($10,500 in calendar year 2000). Under the 401(k) plan, each participant is fully vested in his or her deferred salary contributions. Employee contributions are held and invested by the 401(k) plan's trustee.
Each participant's contributions, and the corresponding investment earnings, are generally not taxable to the participant until withdrawn. Individual participants may direct the trustee to invest their accounts in authorized investment alternatives
Employment Arrangements
We entered into an employment agreement with Dr. Rienhoff in March 1999. Dr. Rienhoff's employment may be terminated at any time by us or Dr. Rienhoff. Under the agreement, Dr. Rienhoff receives a monthly salary of $23,000 for his services as Chairman of the Board and Chief Executive Officer. Dr. Rienhoff is also entitled to the payment of expenses and performance-based cash bonuses as determined by the board of directors. In addition, in the case of the termination of Dr. Rienhoff by us other than for cause, his salary, annual, pro-rated bonus and benefits will continue to be paid for nine months after the date of termination and the vesting of his options and restricted stock will accelerate to the extent that any vesting would otherwise have occurred in the nine months following termination. Cause as defined in the agreement includes conviction of any felony, participation in fraud or an act of dishonesty against us, willful misconduct or gross negligence by Dr. Rienhoff in the performance of his duties or a material breach by Dr. Rienhoff of any element of our Confidential Information and Invention Assignment Agreement. In addition, Dr. Rienhoff has agreed not to carry on any business or activity which is competitive with our business during the nine months following termination.
Mr. Lehrer and Dr. White are entitled to receive nine months severance pay and benefits and six months severance pay and benefits respectively, upon termination of employment without cause. In addition, Mr. Lehrer's options will continue to vest during the nine months following termination without cause. Vesting of Ms. Berland's options will be accelerated by nine months upon any involuntary termination in the two years following a change of control in DNA Sciences.
62
Limitation of Liability and Indemnification
Our certificate of incorporation and bylaws contain provisions permitted under Delaware law relating to the liability of directors. These provisions eliminate a director's personal liability for monetary damages resulting from a breach of fiduciary duty, except in circumstances involving wrongful acts, including:
These provisions do not limit or eliminate our rights or any stockholder's rights to seek non-monetary relief including an injunction or rescission, in the event of a breach of director's fiduciary duty. These provisions will not alter a director's liability under federal securities laws. In addition, we intend to enter into separate indemnification agreements with our directors and officers that provide each of them indemnification protection. We believe that these provisions and agreements will assist us in attracting and retaining qualified individuals to serve as directors and officers.
63
The following is a description of transactions since inception, to which we have been a party in which any director, executive officer or holder of more than 5% of our capital stock had or will have a direct or indirect material interest other than compensation arrangements that are otherwise required to be described under "Management."
Sale of Securities
As of December 29, 2000, we had issued and sold the following shares of common stock and preferred stock:
The following tables sets forth the shares of common stock upon conversion of preferred stock purchased by our officers, directors and holders of more than 5% of our outstanding stock, and their affiliates.
| Name of Purchaser(1) |
Common Stock |
Series A Preferred Stock |
Series B Preferred Stock |
Series C Preferred Stock |
Series C-1 Preferred Stock |
Series D Preferred Stock (2) |
Total Consideration |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monaco Partners, L.P. | 2,000,000 | 2,000,000 | 98,522 | $ | 19,999,998 | ||||||||||
| Entities affiliated with Brentwood Venture Capital | 125,000 | 2,250,000 | 133,334 | 197,043 | 7,500,116 | ||||||||||
| Apple Tree Partners I, L.P.(3) | 1,760,500 | 400,000 | 197,044 | 8,520,997 | |||||||||||
| WebMD Corporation | 1,666,668 | 12,500,010 | |||||||||||||
| Entities affiliated with Quantum Partners | 2,000,000 | 15,000,000 | |||||||||||||
| Entities affiliated with Domain Partners | 2,875,000 | 5,750,000 | |||||||||||||
| Pharmaceutical Product Development, Inc. | 108 | 1,039,267 | 438,565 | 1,478,550 | 36,246,980 | ||||||||||
| Axys Pharmaceuticals, Inc. | 108 | 1,478,550 | 21,246,985 | ||||||||||||
| Hugh Y. Rienhoff, Jr(4) | 1,450,000 | 13,334 | 4,926 | 467,779 | |||||||||||
| Gregory T. Went(4) | 800,000 | 120,000 | 1,060,000 | ||||||||||||
| Raymond White(4) | 200,000 | 300,000 | |||||||||||||
64
We have entered into an amended and restated investors rights agreement with each of the purchasers of preferred stock set forth above, pursuant to which these and other stockholder will have registration rights with respect to their shares of common stock issuable upon conversion of their preferred stock following this offering.
Loans to Executive Officers
We have entered into a security agreement and note on April 12, 1999 with Dr. Rienhoff, under which we loaned Dr. Rienhoff $137,000 for relocation expenses. This loan bears interest at a rate of 5.5% per year compounded annually and is forgivable over a period of four years, provided that Dr. Rienhoff continues to serve as an employee or consultant. Additionally, in connection with the purchase of 325,000 shares of common stock issued pursuant to a restricted stock purchase agreement, we have loaned Dr. Rienhoff $130,000 at an interest rate of 6.2% per year, compounded annually and forgivable over a period of five years, provided that Dr. Rienhoff continues to serve as an employee or consultant.
We have entered into a security agreement and note on December 22, 1999 with Dr. Went, under which we loaned Dr. Went $200,000 for relocation expenses. This loan bears interest at a rate of 6.1% per year compounded annually and is forgivable over a period of five years, provided that Dr. Went continues to serve as an employee or consultant. Additionally, in connection with the purchase of 250,000 shares of common stock issued pursuant to a restricted stock purchase agreement, we have loaned Dr. Went $100,000 at an interest rate of 6.1% per year, compounded annually and forgiveable over a period of five years, provided that Dr. Went continues to serve as an employee or consultant.
We have entered into a security agreement and note on December 30, 1999 with Mr. Lehrer, under which we loaned Mr. Lehrer $300,000 for relocation expenses. This loan bears interest at a rate of 6.2% per year compounded annually and is forgivable over a period of five years, provided that Mr. Lehrer continues to serve as an employee or consultant. Additionally, in connection with the purchase of 150,000 shares of common stock issued pursuant to a restricted stock purchase agreement, we have loaned Mr. Lehrer $60,000 at an interest rate of 6.2% per year, compounded annually and forgiveable over a period of five years, provided that Mr. Lehrer continues to serve as an employee or consultant.
We have entered into a pledge agreement and note on July 27, 2000 with Dr. White, under which we loaned Dr. White $300,000 for relocation expenses at an interest rate of 6.33% per year compounded annually and forgivable over a period of five years, provided that Dr. White continues to serve as an employee or consultant. Dr. Rienhoff has provided a personal guarantee with respect to this loan in the amount of $75,000 for the benefit of DNA Sciences.
Collaboration Agreement with WebMD Corporation
In March 2000, we entered into a collaboration agreement with WebMD to create and host a consumer-oriented genetics channel and a professional-oriented genetics channel on the WebMD website that include information relating to genetic and genomic sciences.
Under this agreement, we are obligated to pay WebMD $3.0 million during the first year of the agreement, $5.0 million in each of the second and third years of the agreement and $10.0 million in each the fourth and fifth years of the agreement. In addition, we are obligated to pay a fee of
65
$2.0 million to WebMD for participating in a launch program under which we were advertised as a new partner of WebMD. We also receive a portion of WebMD's revenues from sales of products or services and from advertising and sponsorship on the portions of the WebMD site or a site provided by WebMD and its portal partners that contains genetic or genomic information provided by us. To date, amounts paid to us under this provision have been immaterial. The initial term of the agreement is five years, and is renewable for additional one-year periods of time. In connection with this agreement, WebMD purchased 1,666,668 shares of Series B preferred stock in March 2000. Upon completion of this offering, WebMD Corporation will beneficially own % of our common stock. For more information on the terms of our agreement with WebMD, see "Business—Strategic Relationships."
PPD Distributor Agreement
Upon the closing of the PPGx acquisition, PPGx amended and restated its distributor agreement with PPD. Under this agreement, PPD was appointed an exclusive distributor of pharmacogenomic products and services developed by PPD or licensed to PPD, except for diagnostic kits or similar products. For more information on PPGx's agreement with PPD, see "Business—PPGx" and "Management's Discussion and Analysis of Financial Condition and Results of Operations—Acquisition of PPGx, Inc."
66
The following table sets forth information with respect to the beneficial ownership of our common stock as of December 29, 2000:
Beneficial ownership of shares is determined under the rules of the Securities and Exchange Commission and generally includes shares over which a person exercises sole or shared voting or investment power. Except as indicated by footnote, and subject to applicable community property laws, each person identified in the table below possesses sole voting power and investment power with respect to all shares of common stock held by them. Shares of common stock subject to options currently exercisable or exercisable within 60 days of December 29, 2000 are deemed outstanding for purposes of calculating the percentage of outstanding shares of the person holding these options, but are not deemed outstanding for purposes of calculating the percentage of any other person. Applicable percentage ownership in the following table is based on 26,193,788 shares of common stock outstanding as of December 29, 2000, after giving effect to the conversion of all outstanding shares of preferred stock into common stock upon the closing of this offering, and shares of common stock outstanding immediately following the completion of this offering.
67
Unless otherwise indicated in the table, the address of each stockholder identified in the table is 6540 Kaiser Dr., Fremont, CA 94555.
| |
|
Percent Beneficially Owned |
|||||
|---|---|---|---|---|---|---|---|
| |
Number of Shares Beneficially Owned |
||||||
| Name and Address of Beneficial Owner |
Prior to Offering |
After Offering |
|||||
| 5% Stockholders | |||||||
| Monaco Partners, L.P 777 East William Street, Suite 200 Carson City, NV 89701 |
3,698,522 | 14.1 | % | ||||
| Pharmaceutical Product Development, Inc. 3151 South 17th Street Wilmington, NC 28412 |
2,956,490 | 11.3 | |||||
| Entities Affiliated with Brentwood Venture Capital(1) 11150 Santa Monica Blvd., Suite 1200 Los Angeles, CA 90025 |
2,705,377 | 10.3 | |||||
| Apple Tree Partners I L.P 41 E. 11th Street, 11th Floor New York, NY 10003 |
2,357,544 | 9.0 | |||||
| Entities Affiliated with Quantum Partners(2) 888 7th Avenue, 33rd Floor New York, NY 10106 |
2,000,000 | 7.6 | |||||
| WebMD Corporation 400 Lenox Building 3399 Peachtree Road, NW Atlanta, GA 30326 |
1,666,668 | 6.4 | |||||
| Entities Affiliated with Domain Partners(3) One Palmer Square, Suite 515 Princeton, NJ 08542 |
1,500,000 | 5.7 | |||||
| Axys Pharmaceuticals, Inc. 180 Kimball Way South San Francisco, CA 94080 |
1,478,658 | 5.6 | |||||
Directors and Named Executive Officers |
|||||||
| Hugh Y. Rienhoff, Jr.(4) | 1,383,935 | 5.3 | |||||
| Steven B. Lehrer(5) | 604,000 | 2.3 | |||||
| Gregory T. Went(6) | 920,000 | 3.5 | |||||
| Ray L. White(7) | 500,000 | 1.9 | |||||
| Brian Atwood(1) | 2,705,377 | 10.3 | |||||
| Walter Burlock(2) | 2,000,000 | 7.6 | |||||
| James Clark(8) | 4,098,522 | 15.6 | |||||
| James D. Watson(9) | 10,025 | * | |||||
| All directors and executive officers as a group (9 persons)(10) |
12,371,859 | 45.9 | |||||
68
the shares beneficially owned by each fund. Mr. Atwood disclaims beneficial ownership of the shares held by each fund within the meaning of Rule 13d-3 under the Securities Exchange Act.
69
Upon the closing of this offering and the filing of our amended and restated certificate of incorporation, our authorized capital stock will consist of 75,000,000 shares of common stock, $0.001 par value, and 5,000,000 shares of preferred stock, $0.001 par value.
Common Stock
As of December 29, 2000, there were 26,193,788 shares of common stock issued and outstanding that were held of record by approximately 95 stockholders, after giving effect to the conversion of our then outstanding preferred stock into common stock which will occur at the closing of this offering. There will be shares of common stock outstanding, assuming no exercise of the underwriters' over-allotment option and no exercise of outstanding options, after giving effect to the sale of the shares of common stock offered by this prospectus and the conversion of shares of preferred stock discussed below.
The holders of common stock are entitled to one vote per share on all matters submitted to a vote of our stockholders. Subject to preferences that may be applicable to any preferred stock outstanding at the time, the holders of outstanding shares of common stock are entitled to receive ratably any dividends out of assets legally available therefor as our board of directors may from time to time determine. Upon our liquidation, dissolution or winding up, holders of our common stock are entitled to share ratably in all assets remaining after payment of liabilities and the liquidation preference of any then outstanding shares of preferred stock. Holders of common stock have no preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to the common stock.
Preferred Stock
Prior to this offering, there were 21,461,014 shares of preferred stock outstanding. All outstanding shares of preferred stock will be converted into 21,461,014 shares of common stock upon the closing of this offering.
Upon the closing of this offering, our certificate of incorporation will provide that our board of directors will have the authority, without further action by our stockholders, to issue up to 5,000,000 shares of preferred stock in one or more series. Our board will be able to fix the rights, preferences, privileges and restrictions of the preferred stock, including dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting any series or the designation of this series. The issuance of preferred stock could adversely affect the voting power of holders of common stock, and the likelihood that holders of preferred stock will receive dividend payments and payments upon liquidation may have the effect of delaying, deferring or preventing a change in control of us, which could depress the market price of our common stock. We have no present plan to issue any shares of preferred stock.
Warrants
As of December 29, 2000, we had the following warrants outstanding:
70
Registration Rights
Upon completion of this offering, under the terms of our Amended and Restated Investor Rights Agreement, the holders of 21,460,689 shares of common stock and warrants to purchase an aggregate of 254,000 shares of common stock are entitled, under certain circumstances, to have the shares under the Securities Act of 1933. If we propose to register any of our securities under the Securities Act, either for our own account or for the account of other security holders, the holders of 21,460,689 shares of common stock and warrants to purchase an aggregate of 254,000 shares of common stock are entitled to notice of the registration and will be entitled to include, at our expense, their shares of common stock. The holders of 21,460,689 shares of common stock and warrants to purchase an aggregate of 200,000 shares of common stock may require us, at our expense and on not more than two occasions, at any time beginning approximately six months from the date of the closing of this offering, to file a registration statement under the Securities Act with respect to their shares of common stock. In such event, we will be required to use our best efforts to effect the registration. In addition, upon completion of this offering, the holders of the 2,683,343 shares of Series C preferred stock and 2,957,100 shares of Series D preferred stock may require us, at our expense, to use our best efforts to effect one additional registration with respect to the shares of common stock into which their shares of preferred stock will be converted. The holders of 21,460,689 shares of common stock and warrants to purchase 200,000 shares of common stock may also require us to register all or a portion of these shares on Form S-3 under the Securities Act when use of this form becomes available to us. All registration rights expire for any particular stockholder following this offering when the holder is able to sell all its shares pursuant to Rule 144 under the Securities Act in any three-month period. In addition, all registration rights are subject to conditions and limitations, including the right of the underwriters of an offering to limit the number of shares to be included in such registration.
Anti-takeover Provisions of Delaware Law and our Charter
We are subject to Section 203 of the Delaware General Corporation Law. In general, the statute prohibits a publicly held Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years following the date that the stockholder becomes an interested stockholder unless:
Section 203 defines "business combination" to include:
71
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Our bylaws provide that candidates for director may be nominated only by the board of directors or by a stockholder who gives written notice to us no later than 60 days prior nor earlier than 90 days prior to the first anniversary of the last annual meeting of stockholders. The board may consist of one or more members to be determined from time to time by the board. The board currently consists of six members divided into three different classes. As a result, only one class of directors will be elected at each annual meeting of our stockholders with the other classes continuing for the remainder of their respective terms. Between stockholder meetings, the board may appoint new directors to fill vacancies or newly created directorships.
Our amended and restated certificate of incorporation requires that upon completion of this offering, any action required or permitted to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and may not be effected by a consent in writing. Upon completion of this offering, our amended and restated certificate of incorporation will also provide that the authorized number of directors may be changed only by resolution of the board of directors. Delaware law and these charter provisions may have the effect of deterring hostile takeovers or delaying changes in control of our management, which could depress the market price of our common stock.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Mellon Investor Services.
72
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock. Future sales of substantial amounts of our common stock in the public market could adversely affect prevailing market prices from time to time. For a period of 180 days or more following this offering, 26,193,788 shares of our common stock will not be freely tradable due to contractual and legal restrictions as described below. Sales of substantial amounts of our common stock in the public market after these restrictions lapse could depress the prevailing market price and limit our ability to raise equity in the future.
Upon closing of this offering, and based on shares outstanding as of December 29, 2000, we will have an aggregate of shares of common stock outstanding, assuming no exercise of the underwriters' over-allotment option and no exercise of outstanding options or warrants. Of the outstanding shares, the shares sold in this offering will be freely tradable, except that any of the shares purchased by our "affiliates," as that term is defined in Rule 144 promulgated under the Securities Act, may only be sold in compliance with the limitations described below. The remaining 26,193,788 shares of common stock held by existing stockholders will be deemed "restricted securities" as defined under Rule 144. Restricted securities may be sold in the public market only if registered or if they qualify for an exemption from registration under Rule 144, including 144(k), or Rule 701 promulgated under the Securities Act, which are summarized below.
As a result of contractual restrictions on transfer and the provisions of Rules 144, including 144(k), and Rule 701, the restricted shares will be available for sale in the public market as follows:
Rule 144
In general, under Rule 144 as currently in effect, beginning 90 days after the date the registration statement of which this prospectus is a part is declared effective, a person or persons whose shares are aggregated, who has beneficially owned restricted securities for at least one year, including the holding period of any prior owner except an affiliate, would be entitled to sell within any three-month period a number of shares that does not exceed the greater of:
Sales under Rule 144 are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us.
Further, under Rule 144(k), a person who has not been one of our affiliates at any time during the 90 days before a sale, and who has beneficially owned the restricted shares for at least two years, is entitled to sell the shares without complying with the manner of sale, public information, volume limitation or notice provisions of Rule 144.
73
Rule 701
In general, under Rule 701 of the Securities Act as currently in effect, any of our employees, consultants or advisors, other than affiliates, who purchase or receive shares from us in connection with a compensatory stock purchase or option plan or other written agreement will be eligible to resell their shares beginning 90 days after the effective date of the registration statement of which this prospectus is a part, subject only to the manner of sale provisions of Rule 144, and by affiliates under Rule 144 without compliance with its holding period requirements.
Registration Rights
Upon completion of this offering, the holders of 21,460,689 shares of our common stock and warrants to purchase an aggregate of 254,000 shares of our common stock, or their transferees, will be entitled to rights with respect to the registration of their shares under the Securities Act. Registration of their shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates, immediately upon the effectiveness of such registration. See "Description of Capital Stock—Registration Rights" above for more information about these rights.
Shares Issued Under Stock Plans
Immediately after this offering, we intend to file a registration statement under the Securities Act covering the shares of common stock reserved for issuance under our 2001 Equity Incentive Plan, 2001 Non-employee Directors' Stock Option Plan and 2001 Employee Stock Purchase Plan. The registration statement is expected to be filed and become effective as soon as practicable after the closing of this offering. Accordingly, shares registered under the registration statements will, subject to Rule 144 volume limitations applicable to affiliates and to any policy we have relating to securities trading by our employees, officers and directors, be available for sale in the open market beginning 180 days after the effective date of the registration statement of which this prospectus is a part.
74
Under the underwriting agreement, which is filed as an exhibit to the registration statement relating to this prospectus, the underwriters named below, for whom Lehman Brothers Inc., CIBC World Markets Corp. and Dain Rauscher Incorporated are acting as representatives, have each agreed to purchase from us the respective number of shares of common stock shown opposite their names below:
| Underwriter |
Number of Shares |
|||
|---|---|---|---|---|
| Lehman Brothers Inc. | ||||
| CIBC World Markets Corp. | ||||
| Dain Rauscher Incorporated | ||||
| Total | ||||
This is a firm commitment underwriting. This means that the underwriters have agreed to purchase all of the shares offered by this prospectus, other than those covered by the over-allotment option described below, if any are purchased. Under the underwriting agreement, if an underwriter defaults in its commitment to purchase shares, the commitments of non-defaulting underwriters may be increased or the underwriting agreement may be terminated, depending on the circumstances.
We plan to apply for quotation of our common stock on the Nasdaq National Market under the symbol "DNAS."
Commissions and Expenses
The representatives of the underwriters have advised us that the underwriters propose to offer the common stock directly to the public at the public offering price on the cover page of this prospectus, and to selected dealers, who may include the underwriters, at the public offering price less a selling concession not in excess of $ per share. The underwriters may allow, and the selected dealers may reallow, a concession not in excess of $ per share to brokers and dealers. After the completion of this offering, the underwriters may change the offering price and other selling terms.
The following table summarizes the underwriting discounts and commissions we will pay to the underwriters. These amounts are shown assuming both no exercise and full exercise of the underwriters' over-allotment option to purchase additional shares. The underwriting discounts and commissions are equal to the public offer price per share, less the amount paid to us per share.
| |
Paid by Us |
|||||
|---|---|---|---|---|---|---|
| |
No Exercise of Over-Allotment Option |
Full Exercise of Over-Allotment Option |
||||
| Per Share | $ | $ | ||||
| Total | ||||||
We estimate that the total expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding underwriting discounts and commissions, will be approximately $ million.
Over-Allotment Option
We have granted to the underwriters an option to purchase up to an aggregate of shares of common stock, exercisable solely to cover over-allotments, if any, at the public offering price less the underwriting discounts and commissions shown on the cover page of this prospectus. The underwriters may exercise this option at any time until 30 days after the date of this prospectus. To the extent the underwriters exercise this option, each underwriter will be committed, so long as the
75
conditions of the underwriting agreement are satisfied, to purchase a number of additional shares proportionate to that underwriter's initial commitment as indicated in the preceding table.
Lock-Up Agreements
We, our directors, officers and other stockholders have agreed not, directly or indirectly, to offer for sale, sell, pledge or otherwise dispose of any common stock or any securities which may be converted into or exchanged for any common stock for a period of 180 days from the date of this prospectus without the prior written consent of Lehman Brothers Inc. In addition, shares acquired in the directed share program by our officers, directors and 5% or greater stockholders will be subject to the lock-up agreements. Lehman Brothers Inc., in its sole discretion, may release the shares subject to the lock-up agreements in whole or in part at any time with or without notice. However, Lehman Brothers Inc. has informed us that it has no current plan to do so.
Offering Price Determination
Prior to this offering, there has been no public market for our common stock. The initial public offering price will be negotiated between the representatives and us. In determining the initial public offering price of our common stock, the representatives will consider:
Indemnification
We have agreed to indemnify the underwriters against liabilities relating to this offering, including liabilities under the Securities Act of 1933, as amended, and liabilities arising from breaches of the representations and warranties contained in the underwriting agreement, and to contribute to payments that the underwriters may be required to make for these liabilities.
Stabilization, Short Positions and Penalty Bids
The representatives may engage in over-allotment, stabilizing transactions, syndicate covering transactions and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of the common stock, in accordance with Regulation M under the Securities Exchange Act of 1934, as amended.
76
The underwriters may purchase and sell shares of common stock in the open market. These transactions may include short sales, stabilizing transactions and purchases to cover positions created by short sales. Short sales involve the sale by the underwriters of a greater number of shares than they are required to purchase in the offering. "Covered" short sales are sales made in an amount not greater than the underwriters' option to purchase additional shares from the issuer in the offering. The underwriters may close out any covered short position by either exercising their option to purchase additional shares or purchasing shares in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through-the over-allotment option. "Naked" short sales are any sales in excess of such option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of common stock made by the underwriters in the open market prior to the completion of the offering.
Similar to other purchase transactions, the underwriters' purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of the common stock or preventing or retarding a decline in the market price of the common stock. As a result, the price of the common stock may be higher than the price that might otherwise exist in the open market.
Neither we nor any of the underwriters make any representation or prediction concerning the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor any of the underwriters make any representation that the representatives will engage in these transactions or that any such transaction, once commenced, will not be discontinued without notice.
Distribution
Lehman Brothers Inc., CIBC World Markets Corp. and Dain Rauscher Incorporated intend to distribute and deliver this prospectus only by hand or mail and intend to use only printed prospectuses.
Stamp Taxes
Purchasers of the shares of our common stock offered by this prospectus may be required to pay stamp taxes and other charges under the laws and practices of the country of purchase, in addition of the offering price listed on the cover of this prospectus.
Offer and Sales in Canada
Any offers in Canada will be made only under an exception from the requirements to file a prospectus in each relevant province of Canada where a sale is made.
Directed Share Program
At our request, Lehman Brothers Inc. has reserved up to shares, or % of our common stock offered by this prospectus, for sale under a directed share program to our officers, directors, employees and to our business associates. All of the persons purchasing the reserved shares must commit to purchase no later than the close of business on the day following the date of this prospectus. The number of shares available for sale to the general public will be reduced to the extent these persons purchase the reserved shares. Shares committed to be purchased by directed share participants which are not so purchased will be reallocated for sale to the general public in the offering.
77
All sales of shares pursuant to the directed share program will be made at the initial public offering price set forth on the cover page of this prospectus.
Sales to Discretionary Accounts
The underwriters have informed us that they do not intend to confirm sales to discretionary accounts that exceed 5% of the total number of shares of our common stock offered by them.
The validity of the common stock offered by this prospectus will be passed upon for us by Cooley Godward LLP, Palo Alto, California. Cooley Godward LLP has a warrant to purchase 200,000 shares of our common stock and an investment partnership associated with Cooley Godward LLP owns 13,334 shares of our common stock. Fried, Frank, Harris, Shriver & Jacobson, a partnership including professional corporations, New York, New York, is acting as counsel to the underwriters in connection with certain legal matters relating to the shares of common stock offered by this prospectus.
Ernst & Young LLP has audited our financial statements at December 31, 1998 and 1999 and for the periods from May 11, 1998 (inception) through December 31, 1998 and 1999 and the year ended December 31, 1999. We have included our financial statements in this prospectus in reliance on Ernst & Young LLP's report given on their authority as experts in accounting and auditing.
Ernst & Young LLP has audited the financial statements of PPGx at December 31, 1999 and for the period from January 25, 1999 (inception) to December 31, 1999. PPGx's financial statements have been included in this prospectus in reliance on Ernst & Young LLP's report given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the Securities and Exchange Commission, Washington, D.C., a registration statement on Form S-1, together with required schedules and exhibits, under the Securities Act with respect to the shares of common stock to be sold in this offering. This prospectus, which constitutes a part of the registration statement, does not contain all of the information provided in the registration statement. We have omitted certain items in accordance with the rules and regulations of the Securities and Exchange Commission. You can find additional information with respect to us and our common stock in the registration statement, which may be inspected without charge, at the public reference facilities maintained by the Securities and Exchange Commission at Judiciary Plaza, 450 Fifth Street, N.W., Washington, D.C. 20549; Seven World Trade Center, 13th Floor, New York, New York 10048; and 500 West Madison Street, Suite 1400, Chicago, Illinois 60661. Copies of these materials may be obtained from the Public Reference Room of the Securities and Exchange Commission, 450 Fifth Street, N.W., Washington, D.C., 20549, at prescribed rates. You may obtain information on the operation of the Public Reference Room by calling the Securities and Exchange Commission at 1-800-SEC-0330. Our SEC filings, including the registration statement, are also publicly available through the Securities and Exchange Commission's website located at http://www.sec.gov.
As a result of this offering, we will become subject to the information and reporting requirements of the Securities Exchange Act of 1934 and, in accordance with those requirements, will file periodic reports, proxy statements and other information with the SEC.
78
DNA SCIENCES, INC.
INDEX TO FINANCIAL STATEMENTS
| |
Page |
|
|---|---|---|
| DNA Sciences, Inc. | ||
| Report of Ernst & Young LLP, Independent Auditors | F-2 | |
| Balance Sheets | F-3 | |
| Statements of Operations | F-4 | |
| Statement of Redeemable Convertible Preferred Stock and Stockholders' Equity (Net Capital Deficiency) | F-5 | |
| Statements of Cash Flows | F-7 | |
| Notes to Financial Statements | F-9 | |
PPGx, Inc. |
||
| Report of Ernst & Young LLP, Independent Auditors | F-26 | |
| Consolidated Balance Sheets | F-27 | |
| Consolidated Statements of Operations | F-28 | |
| Consolidated Statements of Stockholders' Deficit | F-29 | |
| Consolidated Statements of Cash Flows | F-30 | |
| Notes to Consolidated Financial Statements | F-31 | |
Unaudited Pro Forma Combined Condensed Financial Statements |
||
| Unaudited Pro Forma Combined Condensed Balance Sheet as of September 30, 2000 | F-40 | |
| Unaudited Pro Forma Combined Condensed Statement of Operations for the year ended December 31, 1999 | F-41 | |
| Unaudited Pro Forma Combined Condensed Statement of Operations for the nine months ended September 30, 2000 | F-42 | |
| Notes to Unaudited Pro Forma Combined Condensed Financial Statements | F-43 |
F-1
REPORT OF ERNST & YOUNG LLP, INDEPENDENT AUDITORS
To the Board of Directors and Stockholders
DNA Sciences, Inc.
We have audited the accompanying balance sheets of DNA Sciences, Inc. (a development stage company) as of December 31, 1998 and 1999, and the related statements of operations, redeemable convertible preferred stock and stockholders' equity (net capital deficiency), and cash flows for the periods from May 11, 1998 (inception) to December 31, 1998 and 1999 and for the year ended December 31, 1999. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of DNA Sciences, Inc. at December 31, 1998 and 1999, and the results of its operations and its cash flows for the periods from May 11, 1998 (inception) to December 31, 1998 and 1999 and for the year ended December 31, 1999, in conformity with accounting principles generally accepted in the United States.
/s/ ERNST & YOUNG LLP
Palo
Alto, California
March 10, 2000, except for
the first paragraph of Note 9
as to which the date is December 5, 2000
F-2
DNA Sciences, Inc.
(a development stage company)
BALANCE SHEETS
(in thousands except share and per share data)
| |
|
|
|
Unaudited Pro Forma Redeemable Convertible Preferred Stock and Stockholders' Equity as of September 30, 2000 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
As of December 31, |
|
|||||||||||||
| |
As of September 30, 2000 |
||||||||||||||
| |
1998 |
1999 |
|||||||||||||
| |
|
|
(Unaudited) |
(Note 1) |
|||||||||||
| Assets | |||||||||||||||
| Current assets: | |||||||||||||||
| Cash and cash equivalents | $ | 1,077 | $ | 3,483 | $ | 40,882 | |||||||||
| Grant and other receivables | — | 110 | 204 | ||||||||||||
| Prepaid expenses and other current assets | — | 47 | 449 | ||||||||||||
| Total current assets | 1,077 | 3,640 | 41,535 | ||||||||||||
| Property and equipment, net | 30 | 4,215 | 18,079 | ||||||||||||
| Notes receivable—related parties | — | 113 | 900 | ||||||||||||
| Other assets | 43 | 58 | 939 | ||||||||||||
| Total assets | $ | 1,150 | $ | 8,026 | $ | 61,453 | |||||||||
Liabilities and stockholders' equity (net capital deficiency) |
|||||||||||||||
| Current liabilities: | |||||||||||||||
| Accounts payable | $ | 86 | $ | 764 | $ | 3,852 | |||||||||
| Accrued equipment purchase | — | 1,396 | — | ||||||||||||
| Payroll-related liabilities | — | 57 | 169 | ||||||||||||
| Other accrued liabilities | 62 | 148 | 414 | ||||||||||||
| Due to related party | — | — | 2,970 | ||||||||||||
| Deferred revenue | — | 25 | 25 | ||||||||||||
| Notes payable to investors | 1,350 | — | — | ||||||||||||
| Current portion of equipment financing obligations | — | 459 | 2,957 | ||||||||||||
| Total current liabilities | 1,498 | 2,849 | 10,387 | ||||||||||||
| Long-term portion of equipment financing obligations | — | 1,582 | 5,520 | ||||||||||||
| Commitments | |||||||||||||||
| Redeemable convertible preferred stock, $0.001 par value; designated in series; 22,000,000 shares authorized; 5,672,500 and 15,382,006 shares issued and outstanding at December 31, 1999 and September 30, 2000, respectively (none in 1998 or pro forma); aggregate liquidation preference of $11,345 and $69,809 at December 31, 1999 and September 30, 2000, respectively (none pro forma). | — | 11,345 | 69,809 | $ | — | ||||||||||
| Stockholders' equity (net capital deficiency): | |||||||||||||||
| Common stock, $0.001 par value: 40,000,000 shares authorized; 2,279,000 and 4,075,708 shares issued and outstanding at December 31, 1999 and September 30, 2000, respectively (none in 1998) (19,457,714 shares pro forma) | — | 2 | 4 | 19 | |||||||||||
| Additional paid-in capital | — | 3,640 | 22,824 | 92,618 | |||||||||||
| Notes receivable from stockholders | — | (160 | ) | (244 | ) | (244 | ) | ||||||||
| Deferred stock compensation | — | (3,047 | ) | (15,205 | ) | (15,205 | ) | ||||||||
| Deficit accumulated during the development stage | (348 | ) | (8,185 | ) | (31,642 | ) | (31,642 | ) | |||||||
| Total stockholders' equity (net capital deficiency) | (348 | ) | (7,750 | ) | (24,263 | ) | $ | 45,546 | |||||||
| Total liabilities and stockholders' equity (net capital deficiency) | $ | 1,150 | $ | 8,026 | $ | 61,453 | |||||||||
See accompanying notes.
F-3
DNA Sciences, Inc.
(a development stage company)
STATEMENTS OF OPERATIONS
(in thousands except share and per share data)
| |
Period from May 11, 1998 (inception) through December 31, 1998 |
|
Period from May 11, 1998 (inception) through December 31, 1999 |
|
|
Period from May 11, 1998 (inception) through September 30, 2000 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Nine months ended September 30, |
|||||||||||||||||||
| |
Year ended December 31, 1999 |
||||||||||||||||||||
| |
1999 |
2000 |
|||||||||||||||||||
| |
|
|
|
(Unaudited) |
(Unaudited) |
||||||||||||||||
| Grant revenue | $ | — | $ | 79 | $ | 79 | $ | — | $ | 500 | $ | 579 | |||||||||
| Operating expenses: | |||||||||||||||||||||
| Research and development (Note A) | 20 | 4,901 | 4,921 | 3,809 | 14,602 | 19,523 | |||||||||||||||
| General and administrative (Note A) | 309 | 2,700 | 3,009 | 1,995 | 4,652 | 7,661 | |||||||||||||||
| Stock-based compensation | — | 418 | 418 | 129 | 5,985 | 6,403 | |||||||||||||||
| Total operating expenses | 329 | 8,019 | 8,348 | 5,933 | 25,239 | 33,587 | |||||||||||||||
| Loss from operations | (329 | ) | (7,940 | ) | (8,269 | ) | (5,933 | ) | (24,739 | ) | (33,008 | ) | |||||||||
| Interest income (expense), net | (19 | ) | 103 | 84 | 85 | 1,282 | 1,366 | ||||||||||||||
| Net loss | $ | (348 | ) | $ | (7,837 | ) | $ | (8,185 | ) | $ | (5,848 | ) | $ | (23,457 | ) | $ | (31,642 | ) | |||
Basic and diluted net loss per common share |
$ |
— |
$ |
(16.01 |
) |
$ |
(13.43 |
) |
$ |
(16.59 |
) |
||||||||||
| Shares used in computing basic and diluted net loss per common share | — | 489,382 | 435,342 | 1,413,990 | |||||||||||||||||
| Pro forma basic and diluted net loss per common share (unaudited) | $ | (1.49 | ) | $ | (1.56 | ) | |||||||||||||||
| Shares used in computing pro forma basic and diluted net loss per common share (unaudited) | 5,243,896 | 15,011,622 | |||||||||||||||||||
| Note A: | |||||||||||||||||||||
| Excludes noncash charges for stock-based compensation as follows: | |||||||||||||||||||||
| Research and development | $ | — | $ | 239 | $ | 239 | $ | 124 | $ | 3,034 | $ | 3,273 | |||||||||
| General and administrative | — | 179 | 179 | 5 | 2,951 | 3,130 | |||||||||||||||
| Total | $ | — | $ | 418 | $ | 418 | $ | 129 | $ | 5,985 | $ | 6,403 | |||||||||
See accompanying notes.
F-4
DNA Sciences, Inc.
(a development stage company)
STATEMENT OF REDEEMABLE CONVERTIBLE PREFERRED STOCK
AND STOCKHOLDERS' EQUITY (NET CAPITAL DEFICIENCY)
For the period from May 11, 1998 (inception) to September 30, 2000
(in thousands except share and per share data)
| |
Redeemable Convertible Preferred Stock |
|
|
|
|
|
Deficit Accumulated During the Development Stage |
Total Stockholders' Equity (Net Capital Deficiency) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Common Stock |
|
|
|
|||||||||||||||||||||||
| |
Additional Paid-In Capital |
Notes Receivable from Stockholders |
Deferred Stock Compensation |
||||||||||||||||||||||||
| |
Shares |
Amount |
Shares |
Amount |
|||||||||||||||||||||||
| Net loss and comprehensive loss | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | $ | (348 | ) | $ | (348 | ) | |||||||||
| Balance at December 31, 1998 | — | — | — | — | — | — | — | (348 | ) | (348 | ) | ||||||||||||||||
| Issuance of common stock to investors at $0.0005 per share in January 1999 for cash and services rendered, net of repurchases | — | — | 299,000 | — | — | — | — | — | — | ||||||||||||||||||
| Issuance of common stock to consultants upon exercise of stock purchase rights at $0.0005 per share in January 1999 for services rendered | — | — | 287,500 | — | — | — | — | — | — | ||||||||||||||||||
| Issuance of common stock to founders and employees upon exercise of options and stock purchase rights at $0.0005 per share for cash in January and February 1999, net of repurchases | — | — | 812,500 | 1 | — | — | — | — | 1 | ||||||||||||||||||
| Issuance of Series A redeemable convertible preferred stock to investors at $2.00 per share for cash in March 1999 | 5,012,500 | 10,025 | — | — | — | — | — | — | — | ||||||||||||||||||
| Issuance of Series A redeemable convertible preferred stock at $2.00 per share for technology rights in March 1999 | 660,000 | 1,320 | — | — | — | — | — | — | — | ||||||||||||||||||
| Issuance of common stock to employees upon exercise of options and stock purchase rights at $0.20 per share for cash and promissory notes in November and December 1999 | — | — | 880,000 | 1 | 175 | (160 | ) | — | — | 16 | |||||||||||||||||
| Fair market value of stock options and purchase rights granted to consultants | — | — | — | — | 66 | — | — | — | 66 | ||||||||||||||||||
| Deferred compensation related to stock option grants | — | — | — | — | 3,399 | — | (3,399 | ) | — | — | |||||||||||||||||
| Amortization of deferred compensation | — | — | — | — | — | — | 352 | — | 352 | ||||||||||||||||||
| Net loss and comprehensive loss | — | — | — | — | — | — | — | (7,837 | ) | (7,837 | ) | ||||||||||||||||
| Balance at December 31, 1999 (carried forward) | 5,672,500 | $ | 11,345 | 2,279,000 | $ | 2 | $ | 3,640 | $ | (160 | ) | $ | (3,047 | ) | $ | (8,185 | ) | $ | (7,750 | ) | |||||||
See accompanying notes.
F-5
DNA Sciences, Inc.
(a development stage company)
STATEMENT OF REDEEMABLE CONVERTIBLE PREFERRED STOCK
AND STOCKHOLDERS' EQUITY (NET CAPITAL DEFICIENCY) (CONTINUED)
For the period from May 11, 1998 (inception) to September 30, 2000
(in thousands except share and per share data)
| |
Redeemable Convertible Preferred Stock |
|
|
|
|
|
Deficit Accumulated During the Development Stage |
Total Stockholders' Equity (Net Capital Deficiency) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Common Stock |
|
|
|
|||||||||||||||||||||||
| |
Additional Paid-In Capital |
Notes Receivable from Stockholders |
Deferred Stock Compensation |
||||||||||||||||||||||||
| |
Shares |
Amount |
Shares |
Amount |
|||||||||||||||||||||||
| Balance at December 31, 1999 (brought forward) | 5,672,500 | $ | 11,345 | 2,279,000 | $ | 2 | $ | 3,640 | $ | (160 | ) | $ | (3,047 | ) | $ | (8,185 | ) | $ | (7,750 | ) | |||||||
| Issuance of Series A redeemable convertible preferred stock to investors at $2.00 per share for cash in January 2000 (unaudited) | 2,610,500 | 5,221 | — | — | — | — | — | — | — | ||||||||||||||||||
| Issuance of Series B redeemable convertible preferred stock to investors at $7.50 per share for cash in March and April 2000 (unaudited) | 7,027,340 | 52,705 | — | — | — | — | — | — | — | ||||||||||||||||||
| Issuance of Series B redeemable convertible preferred stock to a supplier and a consultant at $7.50 per share for software and services in March and July 2000 (unaudited) | 71,666 | 538 | — | — | — | — | — | — | — | ||||||||||||||||||
| Issuance of common stock to consultants upon exercise of stock purchase rights at $0.20 and $0.75 per share for cash and services rendered in March through July 2000, net of repurchases (unaudited) | — | — | 330,000 | — | 75 | — | — | — | 75 | ||||||||||||||||||
| Issuance of common stock to employees upon exercise of options and stock purchase rights at $0.20 and $0.75 per share for cash and promissory notes in January through September 2000, net of repurchases (unaudited) | — | — | 1,466,708 | 2 | 499 | (130 | ) | — | — | 371 | |||||||||||||||||
| Forgiveness of notes receivable to stockholders (Note 5) (unaudited) | — | — | — | — | — | 46 | — | — | 46 | ||||||||||||||||||
| Fair market value of stock options and warrants granted to consultants and suppliers (unaudited) | — | — | — | — | 3,066 | — | — | 3,066 | |||||||||||||||||||
| Deferred compensation related to stock option grants (unaudited) | — | — | — | — | 15,544 | — | (15,544 | ) | — | — | |||||||||||||||||
| Amortization of deferred compensation (unaudited) | — | — | — | — | — | — | 3,386 | — | 3,386 | ||||||||||||||||||
| Net loss and comprehensive loss (unaudited) | — | — | — | — | — | — | — | (23,457 | ) | (23,457 | ) | ||||||||||||||||
| Balance at September 30, 2000 (unaudited) | 15,382,006 | $ | 69,809 | 4,075,708 | $ | 4 | $ | 22,824 | $ | (244 | ) | $ | (15,205 | ) | $ | (31,642 | ) | $ | (24,263 | ) | |||||||
See accompanying notes.
F-6
DNA Sciences, Inc.
(a development stage company)
STATEMENTS OF CASH FLOWS
(in thousands)
| |
Period from May 11, 1998 (inception) through December 31, 1998 |
|
Period from May 11, 1998 (inception) through December 31, 1999 |
|
|
Period from May 11, 1998 (Inception) through September 30, 2000 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Nine months ended September 30, |
|||||||||||||||||||
| |
Year ended December 31, 1999 |
||||||||||||||||||||
| |
1999 |
2000 |
|||||||||||||||||||
| |
|
|
|
(Unaudited) |
(Unaudited) |
||||||||||||||||
| Operating activities | |||||||||||||||||||||
| Net loss | $ | (348 | ) | $ | (7,837 | ) | $ | (8,185 | ) | $ | (5,848 | ) | $ | (23,457 | ) | $ | (31,642 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||||||||||||
| Depreciation and amortization | — | 373 | 373 | 222 | 2,068 | 2,441 | |||||||||||||||
| Noncash stock compensation | — | 418 | 418 | 129 | 5,985 | 6,403 | |||||||||||||||
| Forgiveness of employee notes | — | 24 | 24 | 18 | 129 | 153 | |||||||||||||||
| Redeemable convertible preferred stock issued for certain technology rights and services | — | 1,320 | 1,320 | 1,320 | 38 | 1,358 | |||||||||||||||
| Change in operating assets and liabilities: | |||||||||||||||||||||
| Grant and other receivables | — | (110 | ) | (110 | ) | — | (94 | ) | (204 | ) | |||||||||||
| Prepaid expenses and other current assets | — | (47 | ) | (47 | ) | (97 | ) | (402 | ) | (449 | ) | ||||||||||
| Notes receivable—related parties | — | (137 | ) | (137 | ) | (137 | ) | (870 | ) | (1,007 | ) | ||||||||||
| Other assets | (43 | ) | (15 | ) | (58 | ) | — | (881 | ) | (939 | ) | ||||||||||
| Accounts payable | 86 | 679 | 765 | 199 | 3,087 | 3,852 | |||||||||||||||
| Payroll-related liabilities | — | 57 | 57 | 45 | 112 | 169 | |||||||||||||||
| Other accrued liabilities | 62 | 86 | 148 | 8 | 266 | 414 | |||||||||||||||
| Due to related party | — | — | — | — | 2,970 | 2,970 | |||||||||||||||
| Deferred revenue | — | 25 | 25 | — | — | 25 | |||||||||||||||
| Net cash used in operating activities | (243 | ) | (5,164 | ) | (5,407 | ) | (4,141 | ) | (11,049 | ) | (16,456 | ) | |||||||||
| Investing activities | |||||||||||||||||||||
| Acquisition of property and equipment | (30 | ) | (3,162 | ) | (3,192 | ) | (2,187 | ) | (9,749 | ) | (12,941 | ) | |||||||||
| Net cash used in investing activities | (30 | ) | (3,162 | ) | (3,192 | ) | (2,187 | ) | (9,749 | ) | (12,941 | ) | |||||||||
| Financing activities | |||||||||||||||||||||
| Proceeds from notes payable | 1,350 | — | 1,350 | — | — | 1,350 | |||||||||||||||
| Proceeds from issuance of redeemable convertible preferred stock | — | 8,675 | 8,675 | 8,675 | 57,926 | 66,601 | |||||||||||||||
| Proceeds from issuance of common stock | — | 17 | 17 | 1 | 446 | 463 | |||||||||||||||
| Borrowings under equipment financing obligations | — | 2,197 | 2,197 | 1,841 | 803 | 3,000 | |||||||||||||||
| Payments on equipment financing obligations | — | (157 | ) | (157 | ) | (29 | ) | (978 | ) | (1,135 | ) | ||||||||||
| Net cash provided by financing activities | 1,350 | 10,732 | 12,082 | 10,488 | 58,197 | 70,279 | |||||||||||||||
| Net increase in cash and cash equivalents | 1,077 | 2,406 | 3,483 | 4,160 | 37,399 | 40,882 | |||||||||||||||
| Cash and cash equivalents at beginning of period | — | 1,077 | — | 1,077 | 3,483 | — | |||||||||||||||
| Cash and cash equivalents at end of period | $ | 1,077 | $ | 3,483 | $ | 3,483 | $ | 5,237 | $ | 40,882 | $ | 40,882 | |||||||||
F-7
| |
Period from May 11, 1998 (inception) through December 31, 1998 |
|
Period from May 11, 1998 (inception) through December 31, 1999 |
|
|
Period from May 11, 1998 (inception) through September 30, 2000 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Nine months ended September 30, |
|||||||||||||||||
| |
Year ended December 31, 1999 |
||||||||||||||||||
| |
1999 |
2000 |
|||||||||||||||||
| |
|
|
|
(Unaudited) |
(Unaudited) |
||||||||||||||
| Supplemental schedule of cash flows information | |||||||||||||||||||
| Interest paid | $ | 21 | $ | 81 | $ | 102 | $ | 35 | $ | 485 | $ | 587 | |||||||
| Schedule of noncash investing and financing activities | |||||||||||||||||||
| Issuance of common stock in exchange for note receivable | $ | — | $ | 160 | $ | 160 | $ | — | $ | 130 | $ | 290 | |||||||
| Accrued equipment purchase | $ | — | $ | 1,396 | $ | 1,396 | $ | — | $ | (1,396 | ) | $ | — | ||||||
| Equipment purchased under financing arrangement | $ | — | $ | — | $ | — | $ | — | $ | 6,611 | $ | 6,611 | |||||||
| Conversion of note payable to convertible preferred stock | $ | — | $ | 1,350 | $ | 1,350 | $ | 1,350 | $ | — | $ | 1,350 | |||||||
| Issuance of preferred stock in exchange for software | $ | — | $ | — | $ | — | $ | — | $ | 500 | $ | 500 | |||||||
| Issuance of common stock in exchange for software development services | $ | — | $ | — | $ | — | $ | — | $ | 468 | $ | 468 | |||||||
| Deferred compensation related to stock options | $ | — | $ | 3,399 | $ | 3,399 | $ | 307 | $ | 15,544 | $ | 18,943 | |||||||
See accompanying notes.
F-8
DNA Sciences, Inc.
(a development stage company)
NOTES TO FINANCIAL STATEMENTS
(Information as of September 30, 2000 and for the nine months ended
September 30, 1999 and 2000 is unaudited)
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
DNA Sciences, Inc. (the "Company"), formerly Kiva Genetics, Inc., was incorporated in the state of Delaware on May 11, 1998. DNA Sciences, Inc. is a genetics discovery company focused on identifying the genetic basis of disease susceptibility, disease progression and response to drug treatment.
Since commencement of operations, the Company's activities have primarily consisted of establishing its offices and research facilities, conducting research and development, recruiting management and technical personnel, and obtaining financing. Accordingly, the Company is considered to be in the development stage at September 30, 2000.
In the course of its development activities, the Company has sustained continuing operating losses and expects such losses to continue for at least the next several years. The Company plans to continue to finance its operations with a combination of equity and debt issuances, government grant funding, and clinical discovery services. The Company's ability to continue as a going concern is dependent upon successful execution of financings and, ultimately, upon achieving profitable operations.
Unaudited Interim Financial Statements
The financial information at September 30, 2000 and for the nine-month periods ended September 30, 1999 and 2000 and for the period from inception to September 30, 2000 is unaudited, but has been prepared on the same basis as the annual financial statements and, in the opinion of management, includes all adjustments (consisting of normal recurring adjustments), that the Company considers necessary for a fair presentation of its financial position at that date and the results of its operations and its cash flows for those periods. Operating results for the interim periods are not necessarily indicative of results that may be expected for any future periods.
Unaudited Pro Forma Information
In January 2001, the board of directors authorized the management of the Company to file a registration statement with the Securities and Exchange Commission (the "SEC") permitting the Company to sell shares of its common stock in an initial public offering ("IPO"). If the IPO is consummated under the terms presently anticipated, all of the redeemable convertible preferred stock outstanding will automatically be converted into common stock. Unaudited pro forma redeemable convertible preferred stock and stockholders' equity at September 30, 2000, as adjusted for the assumed conversion of the preferred stock, is set forth on the balance sheet.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with an initial maturity of three months or less to be cash equivalents. Cash and cash equivalents consists of commercial paper and
F-9
amounts on deposit with commercial banks in checking, interest bearing, and demand money market accounts.
The Company accounts for its investments in marketable securities in accordance with Statement of Financial Accounting Standards No. 115, "Accounting for Certain Investments in Debt and Equity Securities" ("SFAS 115"). To date, all marketable securities have been classified as "available-for-sale" and are carried at market value as determined based upon quoted market prices. Unrealized gains and losses have not been material to date. Realized gains and losses and declines in value judged to be other-than-temporary for available-for-sale securities are included in interest income and have not been significant to date. The cost of securities is based on the specific identification method. Realized gains and losses are computed on a specific identification basis.
Property and Equipment
Property and equipment, including capitalized internally developed software, are stated at cost, less accumulated depreciation. Depreciation is calculated using the straight-line method and the cost is amortized over the estimated useful lives of the respective assets which range from two to five years. Leasehold improvements are amortized using the straight-line method over the estimated useful life of the assets or the term of the lease, whichever is shorter.
Impairment of Long-Lived Assets
In accordance with the provisions of Statement of Financial Accounting Standards No. 121, "Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of" ("SFAS 121"), the Company reviews long-lived assets, including property and equipment, for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. Under SFAS 121, an impairment loss would be recognized when estimated undiscounted future cash flows expected to result from the use of the asset and its eventual disposition is less than its carrying amount. Impairment, if any, is assessed using discounted cash flows. Through September 30, 2000, there have been no such losses.
Software Costs
The Company does not sell or license the rights to use software; accordingly, software development costs, consisting of internally developed software, are accounted for using Statement of Position 98-1, "Accounting for the Costs of Computer Software Developed or Obtained for Internal Use" ("SOP 98-1"). Internal and external costs incurred to develop internal use computer software during the application development stage are capitalized. Application development stage costs generally include software license costs as well as configuration, coding, installation, and testing. Capitalized internal-use software development costs are included in property and equipment and are amortized on a straight-line basis over the estimated useful lives of the related software applications of up to three years.
Costs capitalized in 1999 and during the period ended September 30, 2000 related to internal developed software were approximately $344,000 and $3.8 million, respectively. Amortization of these costs amounted to $0 and $335,000 for the twelve months ended December 31, 1999 and for the nine months ended September 30, 2000, respectively. No costs were capitalized in 1998.
F-10
Revenue Recognition
In 1999, the Company was awarded an Advanced Technology Program grant from the United States Department of Commerce of $2.0 million for a research and development project. The term of the grant is two years. Revenue related to grant agreements is recognized as related research and development expenses are incurred as provided for in the grant agreement.
Research and Development Expenses
Research and development expenses consist of costs incurred for Company-sponsored research and development activities. These costs include direct and research-related overhead expenses, including the costs of identifying and recruiting patients for scientific studies. The costs of maintaining the website, DNA.com, which was developed primarily for patient recruitment purposes, have been included in research and development expense. In addition, fees pursuant to the collaboration agreement with WebMD (see Note 8) are also included in research and development expense. The Company incurred approximately $5.2 million of promotion related costs associated with the recruitment of patients during the period ended September 30, 2000 (none for the period ended December 31, 1999). The Company expenses all research and development costs as such costs are incurred.
Stock-Based Compensation
As permitted by Statement of Financial Accounting Standards No. 123, "Accounting for Stock-Based Compensation" ("SFAS 123"), the Company has elected to account for stock options granted to employees and directors using the intrinsic value method and, accordingly, does not recognize compensation expense for stock options granted to employees with exercise prices equal to the fair value of the underlying common shares.
Options granted to non-employees have been accounted for in accordance with SFAS 123 and Emerging Issues Task Force Issue No. 96-18, "Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring or in Conjunction with Selling, Goods or Services," and are periodically re-measured with the resulting value charged to expense over the period the related services are rendered. Pro forma information required by SFAS 123 is also included in Note 6.
Segment Reporting
The Company applies Statement of Financial Accounting Standards No. 131, "Disclosure about Segments of an Enterprise and Related Information" ("SFAS 131"). SFAS 131 establishes annual and interim reporting standards for an enterprise's operating segments and related disclosures about its products, services, geographic areas, and major customers. The Company has determined that it operates in only one segment.
Comprehensive Income (Loss)
The Company reports comprehensive income in accordance with Statement of Financial Accounting Standards No. 130, "Reporting Comprehensive Income" ("SFAS 130"). SFAS 130 requires unrealized gains or losses on the Company's available-for-sale securities to be included in other comprehensive income (loss) and reported as a separate component of stockholders' equity. Such
F-11
unrealized gains or losses have not been material to date, and accordingly, no separate presentation has been made.
New Accounting Pronouncements
In June 1998, the Financial Accounting Standards Board (the "FASB") issued Statement of Financial Accounting Standards No. 133, "Accounting for Derivative Instruments and Hedging Activities" ("SFAS 133"). SFAS 133 establishes accounting and reporting standards for derivative instruments, including certain derivative instruments embedded in other contracts (collectively referred to as derivatives), and for hedging activities. SFAS 133, as amended by SFAS 137, is effective for all fiscal years beginning after June 15, 2000, with earlier application encouraged. The Company does not currently use derivative instruments and therefore does not expect that the adoption of SFAS 133 will have any impact on its financial position or results of operations.
In December 1999, the SEC issued Staff Accounting Bulletin No. 101, "Revenue Recognition in Financial Statements" ("SAB 101"). SAB 101 summarizes the SEC's views in applying generally accepted accounting principles to revenue recognition. The adoption of SAB 101 had no impact on the Company's historical revenue recognition policy.
In March 2000, the FASB issued FASB Interpretation No. 44, "Accounting for Certain Transactions Involving Stock Compensation" ("FIN 44"), which contains rules designed to clarify the application of APB 25. FIN 44 was effective on July 1, 2000. The adoption of FIN 44 did not have a material effect on the Company's operating results and financial position.
Significant Concentrations
Financial instruments that potentially subject the Company to concentrations of credit risk primarily consist of cash equivalents and marketable securities. The Company invests cash which is not required for immediate operating needs primarily in highly liquid investment grade instruments, which bear minimal risk.
Net Loss Per Share
In accordance with Statement of Financial Accounting Standards No. 128, "Earnings Per Share" ("SFAS 128"), basic net loss per share has been computed using the weighted-average number of shares of common stock outstanding during the period, less the weighted-average number of shares of common stock that are subject to repurchase under the terms of restricted stock agreements. Diluted net loss per share includes the impact of options and warrants to purchase common stock, if dilutive. There is no difference between the basic and diluted net loss per share as the Company incurred a net loss for each period presented. Pro forma basic and diluted net loss per share, as presented in the statement of operations, has been computed as described above and also gives effect to the conversion into common stock of the Company's redeemable convertible preferred stock (using the if-converted method) from the original date of issuance. The redeemable convertible preferred stock will convert to common stock upon the closing of the IPO. The Company had no shares issued and outstanding during 1998.
F-12
The following table presents the computation of basic and diluted and pro forma basic and diluted net loss per share:
| |
|
Nine months ended September 30, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year ended December 31, 1999 |
|||||||||||
| |
1999 |
2000 |
||||||||||
| Net loss (in thousands) | $ | (7,837 | ) | $ | (5,848 | ) | $ | (23,457 | ) | |||
| Basic and diluted: | ||||||||||||
| Weighted-average shares of common stock outstanding | 1,787,366 | 1,719,654 | 3,649,180 | |||||||||
| Less weighted-average shares subject to repurchase | (1,297,984 | ) | (1,284,312 | ) | (2,235,190 | ) | ||||||
| Weighted-average shares used in computing basic and diluted net loss per common share | 489,382 | 435,342 | 1,413,990 | |||||||||
| Basic and diluted net loss per share | $ | (16.01 | ) | $ | (13.43 | ) | $ | (16.59 | ) | |||
| Pro forma: | ||||||||||||
| Shares used above | 489,382 | 1,413,990 | ||||||||||
| Pro forma adjustments to reflect weighted-average effect of the assumed conversion of convertible preferred stock | 4,754,514 | 13,597,632 | ||||||||||
| Shares used in computing pro forma basic and diluted net loss per common share | 5,243,896 | 15,011,622 | ||||||||||
| Pro forma basic and diluted net loss per common share | $ | (1.49 | ) | $ | (1.56 | ) | ||||||
In addition to the redeemable convertible preferred stock, the Company had securities outstanding which could potentially dilute basic earnings per share in the future, but were excluded from the computation of diluted net loss per share, as their effect would have been antidilutive. These outstanding securities consist of the following:
| |
|
At September 30, |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
At December 31, 1999 |
|||||||||
| |
1999 |
2000 |
||||||||
| Stock options/purchase rights | 1,630,500 | 404,000 | 2,441,232 | |||||||
| Warrants to purchase common stock | — | — | 10,000 | |||||||
| Warrants to purchase preferred stock (as-if-converted basis) | — | — | 200,000 | |||||||
| Total | 1,630,500 | 404,000 | 2,651,232 | |||||||
| Weighted-average exercise price of options/purchase rights | $ | 0.20 | $ | 0.20 | $ | 0.59 | ||||
| Weighted-average exercise price of warrants | $ | — | $ | — | $ | 7.50 | ||||
F-13
Property and equipment consisted of the following (in thousands):
| |
December 31, |
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
September 30, 2000 |
||||||||||
| |
1998 |
1999 |
|||||||||
| Machinery and equipment | $ | — | $ | 3,449 | $ | 11,363 | |||||
| Computer equipment and computer software | 7 | 919 | 8,107 | ||||||||
| Furniture and fixtures | — | 46 | 75 | ||||||||
| Leasehold improvements | 23 | 174 | 975 | ||||||||
| 30 | 4,588 | 20,520 | |||||||||
| Less accumulated depreciation and amortization | — | (373 | ) | (2,441 | ) | ||||||
| Property and equipment, net | $ | 30 | $ | 4,215 | $ | 18,079 | |||||
3. EQUIPMENT FINANCING
The Company has entered into various equipment financing arrangements each of which are secured by the underlying equipment financed through the arrangement.
In July 1999 the Company entered into an equipment financing agreement for up to $3.0 million with a financing company. During 1999, the Company financed $2.2 million of equipment purchases structured as loans. The equipment loans are to be repaid over 48 months at interest rates ranging from 13.3% to 13.6%. During the nine month period ended September 30, 2000, the Company financed an additional $803,000 of equipment purchases under this financing agreement at an interest rate of 14.1%.
Future minimum principal payments under the equipment financing arrangement at December 31, 1999 are as follows (in thousands):
| Year ended December 31, | |||||
| 2000 | $ | 459 | |||
| 2001 | 524 | ||||
| 2002 | 599 | ||||
| 2003 | 459 | ||||
| Total minimum payments | $ | 2,041 | |||
In June 2000, the Company entered into an agreement to purchase certain equipment to be used in the Company's genotyping activities. The terms of the agreement require the payment of monthly installments of $249,000 over a period of 32 months as consideration for the equipment. No interest rate was stated in the purchase contract. As such, the book value of the equipment and related installment loan have been recorded at an amount which reflects the present value of the installment payments due, using a discount rate of 14%, or approximately $6.6 million. The Company's incremental borrowing rate was used as the basis to determine the discount rate.
F-14
4. FACILITY LEASES
The Company leases its facility and certain equipment under operating leases. The facility lease was established in October 1998, expires in December 2000 and includes scheduled rent increases. In June 2000, the Company entered into a noncancelable agreement to lease additional facilities for a term of seven years. The lease term payments commenced in December 2000 and monthly payments range from $181,849 to $230,782 through the term of the lease. The scheduled rent increases are recognized on a straight-line basis over the term of the lease.
Minimum annual commitments under all operating leases are as follows (in thousands):
| Year ended December 31, | |||||
| 2000 | $ | 674 | |||
| 2001 | 2,182 | ||||
| 2002 | 2,280 | ||||
| 2003 | 2,378 | ||||
| 2004 | 2,476 | ||||
| 2005 and thereafter | 8,015 | ||||
| $ | 18,005 | ||||
Rent expense under the lease agreements for the period from inception (May 11, 1998) through December 31, 1998, for the year ended December 31, 1999, and the nine month period ended September 30, 1999 and 2000 was approximately $22,000, $550,000, $398,000, and $426,000, respectively.
5. RELATED PARTY NOTES RECEIVABLE
During fiscal year 1999, the Company issued $297,000 of full recourse loans to certain employees, of which $272,740 was outstanding at December 31, 1999 (none at December 31, 1998). These loans bear interest at rates ranging from 5.5% to 6.2% with terms ranging from four to five years. One loan totaling $137,000 was for employee relocation costs and the remaining loans were for the purchase of the Company's common stock and are classified in stockholders' equity.
During the nine month period ended September 30, 2000, the Company issued full recourse loans to certain employees of $130,000 for the purchase of the Company's stock and $870,000 for employee relocation costs. These loans bear interest at rates ranging from 6.1% to 6.4% with terms of five years.
The principal and accrued interest on all employee loans are to be forgiven in varying annual amounts over their respective terms. The loan forgiveness arrangements are subject to continuous employment with the Company. The shares of common stock issued to employees in exchange for the full recourse loans are subject to the Company's lapsing right to repurchase.
F-15
6. REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT)
Authorized Stock
In March 2000, the Company registered with the Delaware Secretary of State its amended and restated articles of incorporation under which the Company is authorized to issue 40 million shares of common stock and 22 million shares of preferred stock.
Common Stock Subject to Repurchase
Common stock issued to certain of the Company's employees and outside vendors are subject to the Company's right of repurchase which lapses over varying periods. From inception (May 11, 1998) through September 30, 2000, the Company's employees purchased 3,325,000 restricted shares of common stock, of which 1,588,718, and 1,979,375 shares were subject to repurchase at a weighted-average price per share of $0.08 and $0.17 at December 31, 1999 and September 30, 2000, respectively.
Redeemable Convertible Preferred Stock
Each share of preferred stock is convertible into common stock on a one-for-one basis (subject to, among other things, adjustment for stock splits and dividends) at the option of the holder or automatically upon a public offering in which aggregate gross proceeds to the Company are at least $20 million, there is a sales price per common share of $11.25 and an aggregate pre-money valuation of at least $80,000,000, or upon approval of the holders of more than 50% of the outstanding shares of Preferred Stock.
Beginning September 8, 2001, the holders of shares of both Series A and Series B convertible preferred stock are entitled to receive dividends, at the rate of $0.16 and $0.60, respectively per share per year out of any assets legally available, prior to and in preference to any declaration or payment of any dividend on the common stock of the Company. Dividends are payable annually when, as, and if declared by the board of directors, and such dividends are cumulative. As of September 30, 2000, no dividends have been declared.
Upon the approval of the majority of the then outstanding shares of Series A and Series B preferred stock, the Company must annually redeem one-third of the Series A and Series B preferred stock beginning no earlier than March 16, 2006. The Series A and Series B preferred stock will be redeemed at $2.00 and $7.50 per share respectively plus all declared but unpaid dividends on such shares.
In the event of any liquidation, dissolution, or winding up of the Company, either voluntary or involuntary, the stockholders of Series A and Series B convertible preferred stock are entitled to receive, prior to and in preference to any distribution of any of the assets of the Company to the stockholders of common stock, an amount equal to the sum of $2.00 and $7.50 for each outstanding share of Series A and Series B preferred stock (as adjusted for any stock dividends, combinations, or splits) respectively, plus any declared but unpaid dividends on such shares. After payment in full of the above amounts, the holders of common stock shall be entitled to be paid out of the remaining net assets of the corporation, on a pro rata basis.
F-16
In the event insufficient funds are available to pay all liquidation preferences, then the net assets of the Company shall be paid ratably to the holders of the Series A and Series B preferred stock, in proportion to their respective liquidation preferences. A merger, consolidation, or sale of all or substantially all of the assets of the Company, in which the existing stockholders of the Company prior to the transaction possess less than 50% of the voting power of the surviving entity (or its parent) immediately after the transaction, shall be deemed to be a liquidation, dissolution, or winding up of the Company.
The holder of each share of preferred stock is entitled to voting rights equal to the number of shares of common stock into which each share of preferred stock could be converted, and has voting rights and powers equal to the voting rights and powers of the shares of common stock.
Convertible preferred stock is issuable in series, with rights and preferences designated by the series. The shares designated and outstanding are as follows (dollars in thousands):
| |
As of December 31, 1999 |
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
As of September 30, 2000 |
|||||||||||
| |
Shares Issued and Outstanding |
|
||||||||||
| |
Redemption/ Liquidation Value |
Shares Issued and Outstanding |
Redemption/ Liquidation Value |
|||||||||
| Redeemable convertible preferred stock: | ||||||||||||
| Series A | 5,672,500 | $ | 11,345 | 8,283,000 | $ | 16,566 | ||||||
| Series B | — | — | 7,099,006 | 53,243 | ||||||||
| Total | 5,672,500 | $ | 11,345 | 15,382,006 | $ | 69,809 | ||||||
2000 Equity Incentive Plan
In March 2000, the Company's board of directors and stockholders adopted and approved the 2000 Equity Incentive Plan (the "Plan"), which replaced the 1998 Incentive Stock Option Plan. Under the Plan the board of directors may issue incentive stock options and/or stock purchase rights to employees, including officers and members of the board of directors who are also employees, and nonqualified stock options to employees, officers, directors, consultants, and advisors of the Company. From March to June 2000, the Company's board of directors authorized an additional 4,500,000 options for grant under the Plan bringing the total shares reserved under both plans to 8,884,500 shares of common stock. Under the Plan, incentive options/rights to purchase the Company's common shares may be granted to employees at prices not lower than fair value at the date of grant, as determined by the board of directors. Nonqualified options or stock purchase rights may be granted to key employees, including directors and consultants, at prices not lower than 85% of fair value at the date of grant, as determined by the board of directors. Options have a term of ten years. Certain options are immediately exercisable, at the discretion of the board of directors. If not immediately exercisable, options/rights generally vest over four to five years (vesting at a rate of 20% or 25% at the end of the first year and monthly thereafter).
F-17
Shares issued pursuant to the exercise of an unvested option/right are subject to the Company's right of repurchase which lapse over periods specified by the board of directors, generally four or five years from the date of grant.
Activity under the Plan is as follows:
| |
|
Options/Rights Outstanding |
||||||
|---|---|---|---|---|---|---|---|---|
| |
Shares Available for future grant |
|||||||
| |
Number of Shares |
Weighted-Average Exercise Price Per Share |
||||||
| Shares authorized | 1,721,000 | — | — | |||||
| Balance at December 31, 1998 | 1,721,000 | — | — | |||||
| Shares authorized | 2,663,500 | — | — | |||||
| Options/rights granted | (3,466,000 | ) | 3,466,000 | $ | 0.15 | |||
| Options/rights exercised | — | (1,835,500 | ) | $ | 0.09 | |||
| Repurchased shares | 505,500 | — | — | |||||
| Balance at December 31, 1999 | 1,424,000 | 1,630,500 | $ | 0.20 | ||||
| Shares authorized | 4,500,000 | — | — | |||||
| Options/rights granted | (2,714,534 | ) | 2,714,534 | $ | 0.64 | |||
| Options/rights exercised | — | (1,657,958 | ) | $ | 0.32 | |||
| Options/rights forfeited | 245,844 | (245,844 | ) | $ | 0.38 | |||
| Repurchased shares | 71,250 | — | $ | 0.09 | ||||
| Balance at September 30, 2000 | 3,526,560 | 2,441,232 | $ | 0.59 | ||||
The weighted average fair value of grants during the year ended December 31, 1999 and the nine months ended September 30, 2000 was $1.14 and $7.46 per share, respectively.
The options/rights outstanding and exercisable under the Plan at December 31, 1999 are as follows:
Options/Rights Outstanding |
Weighted- Average Remaining Contractual Life |
|
|||||
|---|---|---|---|---|---|---|---|
| Exercise Price |
Number Outstanding |
Vested Options/Rights |
|||||
| |
|
(In years) |
|
||||
| $ | 0.20 | 1,630,500 | 9.85 | 55,084 | |||
F-18
The options/rights outstanding and exercisable under the Plan at September 30, 2000 are as follows:
Options/Rights Outstanding |
Weighted- Average Remaining Contractual Life |
|
|||||
|---|---|---|---|---|---|---|---|
| Exercise Price |
Number Outstanding |
Vested Options/Rights |
|||||
| |
|
(In years) |
|
||||
| $ | 0.20 | 708,952 | 9.20 | 124,242 | |||
| $ | 0.75 | 1,732,280 | 9.84 | 342,798 | |||
In 1999 and 2000, the Company issued 701,406 common stock purchase rights and stock options, net of repurchases and cancellations, to non-employees for services rendered at a weighted average exercise price of $0.19. Of this amount, 310,000 stock purchase rights were issued outside of the Plan. Options/rights granted to consultants are periodically revalued as they vest in accordance with SFAS 123 and EITF 96-18 using a Black-Scholes valuation model and the following weighted-average assumptions: estimated volatility of 80%, risk-free interest rates of 6%, no dividend yield, and an expected life of the option/rights equal to the full term, generally 10 years from the date of grant. The resulting compensation expense was $66,000 and $1.6 million for the year ended December 31, 1999 and the nine months ended September 30, 2000, respectively.
Stock Compensation
During the year ended December 31, 1999 and the nine months ended September 30, 2000, in connection with stock option grants to employees, the Company recorded deferred stock compensation totaling approximately $3.4 million and $15.5 million, respectively, representing the difference between the fair value of the common stock for financial reporting purposes and the exercise price of the underlying options. The fair value of the common stock for financial reporting purposes was determined based on business factors underlying the value of the common stock on the date the options were granted, viewed in light of the Company's planned IPO and the expected IPO price per share. Deferred compensation is recorded as a reduction of stockholders' equity (net capital deficiency) and is being amortized over the vesting period of the individual options, generally four or five years, using the attribution method. The Company recorded amortization of deferred stock compensation of $352,000 for the year ended December 31, 1999 and $96,000 and $3.4 million for the nine-month periods ended September 30, 1999 and 2000, respectively.
Pro Forma Information
SFAS 123 requires pro forma information regarding net loss, which has been determined as if the Company accounted for its employee stock options under the fair value method of SFAS 123. The Company estimates the fair value of these options at the date of grant using the minimum value method with the following weighted-average assumptions: risk-free interest rate of 6%; a weighted-average expected life of the option from grant date of 4 years; and a dividend yield of zero.
F-19
For pro forma purposes, the estimated fair value of the Company's stock-based awards to its employees is amortized using the attribution method over the options vesting period. The Company's pro forma information is as follows (in thousands, except per share amounts):
| |
|
Nine months ended September 30, |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year ended December 31, 1999 |
||||||||||
| |
1999 |
2000 |
|||||||||
| |
(In thousands, except per share data) |
||||||||||
| As reported: | |||||||||||
| Net loss | $ | (7,837 | ) | $ | (5,848 | ) | $ | (23,457 | ) | ||
| Net loss per common share | $ | (16.01 | ) | $ | (13.43 | ) | $ | (16.59 | ) | ||
| Pro forma: | |||||||||||
| Net loss | $ | (7,943 | ) | $ | (5,951 | ) | $ | (25,367 | ) | ||
| Net loss per common share | $ | (16.24 | ) | $ | (13.68 | ) | $ | (17.94 | ) | ||
Warrants
In connection with the legal services rendered on behalf of the Company in April 2000, the Company granted warrants to purchase 200,000 shares of Series B preferred stock with an exercise price of $7.50 per share. The warrants are exercisable through April 2004. In the accompanying financial statements, these warrants were valued at approximately $937,000 which was charged to stock compensation expense (general and administrative expense).
In connection with consulting services rendered on behalf of the Company in August 2000, the Company granted warrants to purchase 10,000 shares of common stock with an exercise price of $7.50 per share. The warrants are exercisable through August, 2004. In the accompanying financial statements, these warrants were valued at approximately $51,000 which was charged to stock compensation expense (general and administrative expense).
Reserved Shares
As of December 31, 1999 and September 30, 2000, the Company has reserved shares of common stock for future issuance as follows:
| |
As of December 31, 1999 |
As of September 30, 2000 |
|||
|---|---|---|---|---|---|
| Stock options | 3,054,500 | 5,967,792 | |||
| Warrants | — | 210,000 | |||
| Preferred stock, issued and outstanding | 5,672,500 | 15,382,006 | |||
| 8,727,000 | 21,559,798 | ||||
F-20
7. INCOME TAXES
The Company had no provision for U.S. federal or State income taxes for any period as it has incurred operating losses in all periods and for all jurisdictions.
As of December 31, 1999, the Company had federal and state net operating loss carryforwards of approximately $6.4 million and $6.3 million, respectively. The Company also had federal research and development tax credit carryforwards of approximately $100,000. The net operating loss and credit carryforwards will expire at various dates beginning in the year 2006 through 2019, if not utilized.
Utilization of the net operating losses and credits may be subject to a substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986 and similar state provisions. The annual limitation may result in the expiration of net operating losses and credits before utilization. The Company has not calculated the amount of the limitation, if any.
Significant components of the Company's deferred tax assets are as follows (in thousands):
| |
December 31 |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
1998 |
1999 |
||||||
| Net operating loss carry forwards | $ | 150 | $ | 2,600 | ||||
| Research and other credit carry forwards | — | 100 | ||||||
| Acquired technology rights | — | 500 | ||||||
| Total deferred tax assets | 150 | 3,200 | ||||||
| Valuation allowance for deferred tax assets | (150 | ) | (3,200 | ) | ||||
| Net deferred tax assets | $ | — | $ | — | ||||
Because of the Company's lack of earnings history, the deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by approximately $150,000 and $3.1 million during the periods ended December 31, 1998 and 1999, respectively.
8. COLLABORATION AGREEMENT WITH WEBMD CORPORATION
In March 2000, the Company entered into a collaboration agreement with WebMD to create and provide genomic content to the WebMD website. Included in this agreement, the Company has an exclusive right to provide genetic information for commercial purposes to WebMD and WebMD has agreed to provide a specified amount of advertising time on the WebMD website on behalf of the Company. The Company is obligated to pay WebMD $5.0 million in each of the first three years of the agreement, and $10 million in each of the fourth and fifth years. Such amounts are recognized as expense over the period in proportion to the level of promotional efforts by WebMD. The Company is entitled to receive a portion of WebMD's revenues from sales of products, services and advertising on the portions of WebMD's website that contains genomic information. No material revenue has been earned to date. In connection with this agreement, WebMD made a $12.5 million investment in the Company's Series B preferred stock offering.
F-21
During the nine month period ended September 30, 2000, the Company accrued fees of $3.0 million pursuant to this agreement. No payments have been made to date to WebMD and the accrued amounts are reflected as due to related party in the accompanying balance sheet.
9. SUBSEQUENT EVENTS
Stock Split
In November 2000, the Company's board of directors approved a two-for-one stock split, effective December 5, 2000, of all outstanding preferred and common shares. All references to shares, options and warrants outstanding and per share amounts have been adjusted retroactively to reflect the impact of the stock split.
Initial Public Offering
In January 2001, the board of directors approved the filing of a Registration Statement with the Securities and Exchange Commission permitting the Company to sell common stock to the public. Concurrent with the completion of the IPO, the Company's certificate of incorporation will be amended to authorize 75,000,000 shares of common stock and 5,000,000 shares of preferred stock, and all outstanding shares of redeemable convertible preferred stock will convert to common stock.
Option Grants
In the period from October 1, 2000 through December 31, 2000, the Company granted employees additional stock options to purchase 1,385,500 shares of common stock with an exercise price of $0.75 per share and 1,066,416 shares of common stock with an exercise price of $2.05 per share. The Company will record additional deferred stock compensation of approximately $22.3 million in the quarter ending December 31, 2000 to account for the difference between the exercise price of these employees grants and the fair value of the common stock on the date of grant. Such amount will be amortized to expense over the vesting period of the options.
In the period from October 1, 2000 through December 31, 2000, the Company granted options to non-employees to purchase 122,874 shares of common stock at an exercise price of $2.05 per share. These options will be valued using the Black-Scholes model as they vest with resulting value charged to expense.
2001 Employee Stock Purchase Plan
In January 2001, the Company's board of directors approved the adoption of the 2001 Employee Stock Purchase Plan (the "Purchase Plan"), to become effective upon the first day of trading on Nasdaq of the shares being offered in this IPO. Under the terms of the Purchase Plan, eligible employees will be permitted to purchase common stock at 85% of the fair market value of the common stock on the first day or last day of a defined offering period, whichever is lower. A total of 500,000 shares of common stock has been reserved for issuance under the Purchase Plan. On each January 1, starting in 2002 and ending in 2011, the number of shares reserved under the Purchase Plan will be
F-22
automatically increased by the least of (i) 3% of the total outstanding shares of common stock calculated on a fully diluted basis each January 1; (ii) 600,000 shares; or (iii) such smaller number of shares as determined by the board of directors.
2001 Equity Incentive Plan
In January 2001, the Company's board of directors approved the adoption of the 2001 Equity Incentive Plan ("the 2001 Plan"), which amends and restates the 2000 Equity Incentive Plan. The incentive plan will become effective on the closing of the IPO of the Company's common stock and authorizes 9,000,000 shares of common stock for issuance. However, on each January 1, starting with January 1, 2002 and ending on January 1, 2010, the aggregate number of the shares of common stock that may be issued under the incentive plan automatically will be increased by the least of (i) 5% of the total outstanding shares of common stock, determined on each January 1 and calculated on a fully diluted basis; (ii) 2,500,000 shares; or (iii) a smaller number of shares determined by the Company's board of directors.
Shares subject to stock awards that have expired or otherwise terminated without having been exercised in full again become available for the grant of awards under the 2001 Plan. The other terms of the 2001 Plan are similar to the 2000 Equity Incentive Plan.
2001 Non-Employee Directors' Stock Option Plan
In January 2001, the Company's board of directors approved the 2001 Non-employee Directors' Stock Option Plan (the "Directors' Plan"), to become effective upon the closing of the IPO. The Directors' Plan provides for the automatic grant of nonstatutory stock options to the Company's non-employee directors to purchase common stock at an exercise price equal to the fair market value of the stock on the date of the grant. A total of 340,000 shares of common stock has been reserved for issuance under the Directors' Plan.
Equipment Line
In October 2000, the Company entered into two separate credit facilities which provide up to an aggregate of $8.0 million for the purchase of equipment, tenant improvements and certain software costs. Approximately $6.2 million has been advanced to date under the credit facilities. The credit agreements bear interest at rates between 12.8% to 14.2% and are payable in monthly installments over 36 months.
Also in connection with one of the credit facilities, the Company issued warrants to purchase 54,000 shares of Series B redeemable preferred stock at an exercise price of $7.50 per share.
Series C and Series C-1 Redeemable Convertible Preferred Stock
In December 2000, the Company issued 2,683,343 shares of Series C redeemable convertible preferred stock ("Series C") and 438,565 shares of Series C-1 redeemable convertible preferred stock at a purchase price of $10.15 per share. The Series C-1 preferred stock is non-voting stock. Each share
F-23
of Series C and Series C-1 preferred stock is convertible into common stock on a one-for-one basis (subject to, among other things, adjustment for stock splits and dividends) at the option of the holder automatically upon a public offering in which aggregate gross proceeds to the Company are at least $40 million and an aggregate pre-money valuation of at least $350 million or upon the approval of the holders of more than 50% of the outstanding shares of preferred stock; provided, however, the Series C-1 preferred stock is only convertible to common stock if the conversion of the Series C-1 does not require any filing with any U.S. governmental authority under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended (the "HSR Act"), or upon the expiration or termination of any applicable waiting period required by a filing under the HSR Act. Each share of Series C-1 preferred stock is convertible into Series C preferred stock when the conversion may be effected without making any filing under the HSR Act, or upon the expiration or termination of any applicable waiting period required by a filing under the HSR Act.
Strategic Relationships
In December 2000, the Company entered into a genetic research agreement with the University of Utah under which the Company has non-exclusive access to the Utah Population Database to identify potential participants in genetic studies. The Company received an exclusive right for five years to use any tissue samples obtained under the collaboration to perform research, an option to obtain an exclusive license for any University or joint inventions made pursuant to the research collaboration, and a right of first negotiation to obtain a license for any other inventions made by University researchers made through the use of tissue samples outside the scope of this collaboration. The Company must pay annual license fees for use of the tissues in connection with any particular task order, and milestone payments and royalties on sales of any products developed using tissue samples acquired from the University. In addition, the Company intends to fund research related to these activities at the University over the term of the agreement. The Company's current financial commitments under this arrangement are not material.
In December 2000, the Company entered into a three year research, development and commercialization agreement with Amersham Pharmacia Biotech Inc. to develop the Company's microchannel plate technology for use in performing DNA sequencing and genotyping studies. Under the agreement, Amersham must provide technology access fees aggregating $7.5 million and funding to support the Company's development efforts based upon research plans to be developed by a joint steering committee. Research funding will be up to $6.3 million depending on the number and location of the employees involved in the research. Additionally, Amersham must sell to the Company reagents at predetermined reduced prices for use in any DNA sequencing and genotyping which is outside the scope of the Company's development of the microchannel plate platform. The Company also has the right to purchase from Amersham some prototype and initial commercial versions of the device developed with the technology.
Acquisition of PPGx
On December 22, 2000, the Company completed the acquisition of all the outstanding equity of PPGx Inc. ("PPGx"). As consideration for the acquisition, the Company issued approximately 2,963,000
F-24
shares of the Company's common stock and Series D redeemable convertible preferred stock with a fair value for accounting purposes of $11.50 per share. The Company also assumed options to purchase approximately 1.4 million shares of PPGx common stock which converted, through the exchange ratio specified in the merger agreement, into options to purchase approximately 306,000 shares of the Company's common stock with a weighted average exercise price of $9.20 per share. The options were valued using the Black-Scholes model. The fair value of such options has been included as part of the cost of the acquisition, net of approximately $449,000 of intrinsic value of unvested options which has been recorded as deferred stock compensation, and will be amortized to expense over the vesting terms of the options. The acquisition of PPGx was accounted for as a purchase, with the results of PPGx's operations included in the Company's results of operations from the date of acquisition.
Based upon a preliminary valuation of tangible and intangible assets acquired, the Company has allocated the excess of the $36.5 million acquisition cost over the fair value of the tangible net assets acquired as follows (in thousands):
| In process research and development | $ | 1,700 | ||
| Assembled workforce | 1,100 | |||
| Developed and core technology | 6,500 | |||
| DNA samples | 15,600 | |||
| Goodwill and other intangibles | 10,540 |
The above amounts are subject to change based on the closing balance sheet of PPGx as of the date of the acquisition and upon the completion of valuation procedures, including an independent asset valuation. The in-process research and development will be charged to expense in the year ended December 31, 2000. The Company expects to amortize the tangible and intangible assets as follows:
| |
Years |
|
|---|---|---|
| Assembled workforce | 2 | |
| Developed and core technology | 5 | |
| DNA samples | 3 | |
| Goodwill and other intangibles | 5 |
Unaudited pro forma information as if the acquisition of PPGx had occurred on January 1, 1999, is as follows (in thousands, except per share amounts):
| |
Year ended December 31, 1999 |
Nine months ended September 30, 2000 |
|||||
|---|---|---|---|---|---|---|---|
| Revenue | $ | 966 | $ | 1,672 | |||
| Net loss | $ | (23,272 | ) | $ | (36,831 | ) | |
| Net loss per common share, basic and diluted | $ | (46.95 | ) | $ | (25.93 | ) | |
The unaudited pro forma information is presented for illustrative purposes only and is not necessarily indicative of the operating results that would have occurred had the transaction been completed at the beginning of the earliest period presented, nor is it necessarily indicative of future operating results.
F-25
REPORT OF ERNST & YOUNG LLP, INDEPENDENT AUDITORS
To the Board of Directors and Stockholders
PPGx, Inc.
We have audited the accompanying consolidated balance sheets of PPGx, Inc. as of December 31, 1999, and the related consolidated statements of operations, stockholders' deficit and cash flows for the period from January 25, 1999 (inception) through December 31, 1999. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of PPGx, Inc. at December 31, 1999, and the consolidated results of its operations and its cash flows for the period from January 25, 1999 (inception) through December 31, 1999, in conformity with accounting principles generally accepted in the United States.
As discussed in Note 1 to the consolidated financial statements, the Company's deficiency in working capital, stockholders' deficit, and loss from operations raise substantial doubt about its ability to continue as a going concern. Management's plans as to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ ERNST & YOUNG LLP
San
Diego, California
March 3, 2000
F-26
PPGx, Inc.
CONSOLIDATED BALANCE SHEETS
(in thousands except share data)
| |
December 31, 1999 |
September 30, 2000 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| |
|
(unaudited) |
|||||||
| Assets | |||||||||
| Current assets: | |||||||||
| Cash and cash equivalents | $ | 2,190 | $ | 352 | |||||
| Due from related party | 211 | 394 | |||||||
| Other current assets | 81 | 164 | |||||||
| Total current assets | 2,482 | 910 | |||||||
| Property and equipment, net | 1,611 | 1,394 | |||||||
| Other assets, net | 1,389 | 889 | |||||||
| Total assets | $ | 5,482 | $ | 3,193 | |||||
Liabilities and stockholders' deficit |
|||||||||
| Current liabilities: | |||||||||
| Accounts payable and accrued expenses | $ | 259 | $ | 412 | |||||
| Accrued compensation | 474 | 538 | |||||||
| Other current liabilities | 128 | 324 | |||||||
| Due to related parties | 173 | 174 | |||||||
| Notes payable to related parties | — | 700 | |||||||
| Note payable | 6,000 | 9,000 | |||||||
| Total current liabilities | 7,034 | 11,148 | |||||||
| Commitments | |||||||||
| Stockholders' deficit: | |||||||||
| Preferred stock, $.001 par value, 10,000,000 shares authorized, issued and outstanding at December 31, 1999 and September 30, 2000 | 10 | 10 | |||||||
| Common stock, $.001 par value, 15,000,000 shares authorized, 1,000 and 26,000 shares issued and outstanding at December 31, 1999 and September 30, 2000, respectively | — | — | |||||||
| Additional paid-in capital | 4,606 | 4,656 | |||||||
| Accumulated other comprehensive loss | (3 | ) | (35 | ) | |||||
| Accumulated deficit | (6,165 | ) | (12,586 | ) | |||||
| Total stockholders' deficit | (1,552 | ) | (7,955 | ) | |||||
| Total liabilities and stockholders' deficit | $ | 5,482 | $ | 3,193 | |||||
See accompanying notes.
F-27
PPGx, Inc.
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands)
| |
For the period from January 25, 1999 (inception) through December 31, 1999 |
For the period from January 25, 1999 (inception) through September 30, 1999 |
Nine months ended September 30, 2000 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(unaudited) |
|||||||||||
| Revenues | $ | 887 | $ | 646 | $ | 1,172 | ||||||
| Cost of revenues | 1,030 | 750 | 1,243 | |||||||||
| Gross margin | (143 | ) | (104 | ) | (71 | ) | ||||||
| Expenses: | ||||||||||||
| Research and development | 2,121 | 1,461 | 2,506 | |||||||||
| General and administrative | 3,818 | 2,591 | 3,457 | |||||||||
| Total operating expenses | 5,939 | 4,052 | 5,963 | |||||||||
| Operating loss | (6,082 | ) | (4,156 | ) | (6,034 | ) | ||||||
| Interest and other expense, net | (83 | ) | (25 | ) | (387 | ) | ||||||
| Net loss | $ | (6,165 | ) | $ | (4,181 | ) | $ | (6,421 | ) | |||
See accompanying notes.
F-28
PPGx, Inc.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' DEFICIT
(in thousands except share and per share data)
| |
Preferred Stock |
Common Stock |
|
|
|
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Additional Paid-in Capital |
Accumulated Other Comprehensive Loss |
Accumulated Deficit |
Total Stockholders' Deficit |
|||||||||||||||||||
| |
Shares |
Amount |
Shares |
Amount |
|||||||||||||||||||
| Issuance of Series A Preferred stock and common stock at $.001 par value per share for cash and assets in February 1999 | 10,000,000 | $ | 10 | 1,000 | — | $ | 4,606 | $ | — | $ | — | $ | 4,616 | ||||||||||
| Translation adjustment | — | — | — | — | — | (3 | ) | — | (3 | ) | |||||||||||||
| Net loss | — | — | — | — | — | — | (6,165 | ) | (6,165 | ) | |||||||||||||
| Balance at December 31, 1999 |
10,000,000 | 10 | 1,000 | — | 4,606 | (3 | ) | (6,165 | ) | (1,552 | ) | ||||||||||||
| Issuance of common stock at $0.001 par value (unaudited) | — | — | 25,000 | — | 50 | — | — | 50 | |||||||||||||||
| Translation adjustment (unaudited) | — | — | — | — | — | (32 | ) | — | (32 | ) | |||||||||||||
| Net loss (unaudited) | — | — | — | — | — | — | (6,421 | ) | (6,421 | ) | |||||||||||||
| Balance at September 30, 2000 (unaudited) | 10,000,000 | $ | 10 | 26,000 | — | $ | 4,656 | $ | (35 | ) | $ | (12,586 | ) | $ | (7,955 | ) | |||||||
See accompanying notes.
F-29
PPGx, Inc.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| |
For the period from January 25, 1999 (inception) through December 31, 1999 |
For the period from January 25, 1999 (inception) through September 30, 1999 |
Nine months ended September 30, 2000 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
(unaudited) |
||||||||||
| Operating activities | ||||||||||||
| Net loss | $ | (6,165 | ) | $ | (4,181 | ) | $ | (6,421 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation and amortization | 1,057 | 723 | 952 | |||||||||
| Loss on sales of property and equipment | 23 | 23 | — | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Other assets | (49 | ) | (108 | ) | (83 | ) | ||||||
| Due to/from related parties | 114 | 22 | (182 | ) | ||||||||
| Accounts payable and accrued expenses | 259 | 275 | 154 | |||||||||
| Accrued compensation | 424 | 393 | 65 | |||||||||
| Other current liabilities | 128 | 46 | 196 | |||||||||
| Net cash used in operating activities | (4,209 | ) | (2,807 | ) | (5,319 | ) | ||||||
| Investing activities | ||||||||||||
| Purchases of property and equipment | (1,164 | ) | (834 | ) | (235 | ) | ||||||
| Proceeds from sales of property and equipment | 60 | 60 | — | |||||||||
| Net cash used in investing activities | (1,104 | ) | (774 | ) | (235 | ) | ||||||
| Financing activities | ||||||||||||
| Proceeds from issuance of preferred stock and common stock | 1,500 | 1,500 | 50 | |||||||||
| Proceeds from notes payable to related parties | — | — | 700 | |||||||||
| Proceeds from notes payable | 6,000 | 2,500 | 3,000 | |||||||||
| Net cash provided by financing activities | 7,500 | 4,000 | 3,750 | |||||||||
| Net decrease in cash and cash equivalents | 2,187 | 419 | (1,804 | ) | ||||||||
| Effect of exchange rate change on cash and cash equivalents | 3 | (3 | ) | (34 | ) | |||||||
| Cash and cash equivalents at beginning of period | — | — | 2,190 | |||||||||
| Cash and cash equivalents at end of period | $ | 2,190 | $ | 416 | $ | 352 | ||||||
| Supplemental schedule of noncash operating activities | ||||||||||||
| Other assets acquired in exchange for preferred stock and common stock | $ | 33 | $ | 33 | $ | — | ||||||
| Due to related party in exchange for preferred stock and common stock | $ | 154 | $ | 154 | $ | — | ||||||
| Accrued compensation and other liabilities in exchange for preferred stock and common stock | $ | 50 | $ | 50 | $ | — | ||||||
| Supplemental schedule of noncash investing and financing activities | ||||||||||||
| Equipment and other assets acquired in exchange for preferred stock and common stock | $ | 2,978 | $ | 2,978 | $ | — | ||||||
| Supplemental disclosure of cash flow information | ||||||||||||
| Interest paid | $ | 26 | $ | 51 | $ | 420 | ||||||
See accompanying notes.
F-30
PPGx, Inc.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Information subsequent to December 31, 1999 and pertaining to September 30,
1999 and the period from
January 25, 1999 (inception) through September 30,
1999, and the nine months ended September 30, 2000 is unaudited)
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization and Business
PPGx, Inc. (the "Company") is a privately held company incorporated on January 25, 1999, and is engaged in the discovery, development and rapid commercialization of innovative proprietary pharmacogenomic products and services for the purposes of tailoring ethical and consumer pharmaceuticals to patient specific genetic variability. The Company maintains operations in the United States and United Kingdom.
The Company was formed as a joint venture between Axys Pharmaceuticals, Inc. ("Axys") and Pharmaceutical Product Development, Inc. ("PPD"), which as of December 31, 1999, own 82% and 18%, respectively, of the outstanding preferred and common stock of the Company. Tangible and intangible net assets contributed to the Company by its stockholders were accounted for at the historical cost basis of the assets and liabilities.
Basis of Consolidation
The accompanying financial statements include the accounts of the Company and its subsidiary. All significant intercompany accounts and transactions have been eliminated in consolidation.
Basis of Presentation
In the period from inception through September 30, 2000, the Company has incurred losses totaling $12,585,977. The Company's ability to transition from the research and development stage and, ultimately, to attain profitable operations is dependent upon obtaining sufficient working capital to complete the successful development of its services and technology, achieving market acceptance of such technology and achieving sufficient levels of revenue to support the Company's cost structure.
The accompanying financial statements have been prepared on a going concern basis. The Company is in the early stages of developing its services and products, and as a result has minimal revenues and losses from operations. The Company's future success is dependent upon the availability of sufficient financing to fund marketing and other infrastructure costs until market acceptance of its services can be achieved. Management of the Company is actively pursuing funding through sales of the Company's capital stock.
The accompanying financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the possible inability of the Company to continue as a going concern.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with a remaining maturity of 90 days or less when purchased to be cash equivalents. Cash equivalents consist principally of a money market account at December 31, 1999 and September 30, 2000.
F-31
Property and Equipment
Property and equipment is stated at cost and depreciated over the estimated useful lives of the assets (3 to 7 years) using the straight-line method. Leasehold improvements are depreciated over the shorter of estimated useful life or lease term using the straight-line method.
Impairment of Long-Lived Assets
In accordance with Statement of Financial Accounting Standards ("SFAS") No. 121, Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of, if indicators of impairment exist, the Company assesses the recoverability of the affected long-lived assets by determining whether the carrying value of such assets can be recovered through undiscounted future operating cash flows. If impairment is indicated, the Company measures the amount of such impairment by comparing the flows associated with the use of the asset. While the Company's current and historical operating and cash flow losses are indicators of impairment, the Company believes the future cash flows to be received from the long-lived assets will exceed the assets' carrying value, and accordingly the Company has not recognized any impairment losses through September 30, 2000.
Software License
Software license, included in other assets, is stated at the historical cost basis of the stockholder which contributed it to the Company. The license is being amortized over the estimated useful life of the asset (three years) using the straight-line method.
Revenue Recognition
The Company recognizes product and related services revenues at the time of the shipment of a product or performance of services to a credit worthy customer. Such amounts are recorded after revenue sharing in accordance with the distribution agreement with PPD, the Company's minority stockholder.
Research and Development Expenses
All costs of research and development are expensed in the period incurred.
Stock-Based Compensation
As permitted by SFAS No. 123, Accounting for Stock-Based Compensation, the Company has elected to follow Accounting Principles Board Opinion No. 25, Accounting for Stock Issued to Employees ("APB 25"), and related Interpretations in accounting for stock-based employee compensation. Under APB 25, if the exercise price of the Company's employee and director stock options equals or exceeds the estimated fair value of the underlying stock on the date of grant, no compensation expense is recognized.
F-32
When the exercise price of the employee or director stock options is less than the estimated fair value of the underlying stock on the grant date, the Company records deferred compensation for the difference and amortizes this amount to expense in accordance with FASB Interpretation No. 28 over the vesting period of the options.
Options or stock awards issued to non-employees are recorded at their fair value as determined in accordance with SFAS No. 123 and EITF 96-18 and recognized over the related service period. Deferred charges for options granted to non-employees are periodically remeasured as the options vest.
Comprehensive Loss
SFAS No. 130, Reporting Comprehensive Income, requires that all components of comprehensive loss, including net loss, be reported in the financial statements in the period in which they are recognized. Comprehensive loss is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Net loss and other comprehensive income or loss, including foreign currency translation adjustments, and unrealized gains and losses on investments, shall be reported, net of their related tax effect, to arrive at comprehensive loss.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Effect of New Accounting Standards
Effective January 1, 2001, the Company will adopt SFAS No. 133, Accounting for Derivative Instruments and Hedging Activities. The adoption of SFAS No. 133 is not anticipated to have an impact on the Company's results of operations or financial condition as the Company holds no derivative financial instruments and does not currently invest in derivative investments or engage on hedging activities.
In March 2000, the Financial Accounting Standards Board issued Financial Interpretation No. 44, or Fin 44, Accounting for Certain Transactions Involving Stock Compensation—an interpretation of APB Opinion No. 25. FIN 44 clarifies the definition of an employee for purposes of applying Accounting Practice Board Opinion No. 25, Accounting for Stock Issued to Employees (APB 25), the criteria for determining whether a plan qualifies as a noncompensatory plan, the accounting consequence of various modifications to the terms of previously fixed stock option or award, and the accounting for an exchange of stock compensation awards in a business combination. FIN 44 became effective on July 1, 2000, but certain conclusions in FIN 44 cover specific events that occur after either December 15, 1998 or January 12, 2000. The adoption of FIN 44 had not had a material effect on the financial position or results of operations of the Company.
F-33
Reclassifications
Certain amounts in the prior year financial statements have been reclassified to conform with the current year presentation.
2. PROPERTY AND EQUIPMENT
Property and equipment consist of the following (in thousands):
| |
December 31, 1999 |
September 30, 2000 |
||||||
|---|---|---|---|---|---|---|---|---|
| Machinery and equipment | $ | 1,201 | $ | 962 | ||||
| Computers and software | 636 | 784 | ||||||
| Furniture and fixtures | 117 | 435 | ||||||
| Leasehold improvements | 87 | 84 | ||||||
| 2,041 | 2,265 | |||||||
| Less accumulated depreciation | (430 | ) | (871 | ) | ||||
| $ | 1,611 | $ | 1,394 | |||||
3. OTHER ASSETS
Other assets consist of the following (in thousands):
| |
December 31, 1999 |
September 30, 2000 |
||||||
|---|---|---|---|---|---|---|---|---|
| Software license | $ | 2,000 | $ | 2,000 | ||||
| Less accumulated amortization | (611 | ) | (1,111 | ) | ||||
| $ | 1,389 | $ | 889 | |||||
4. NOTES PAYABLE
In February 1999 the Company entered into a line of credit agreement with a bank which provides for borrowings of up to $8.0 million. In 2000, this agreement was modified which increased the allowable borrowings to $9.0 million. The line of credit is guaranteed by PPD, a significant stockholder of the Company, and is being renewed on a month to month basis. The line of credit bears interest at LIBOR plus 0.625% (7.335% - 7.3450% at September 30, 2000) or prime plus 2%. As of September 30, 1999, December 31, 1999, and September 30, 2000, $2.5, $6.0 and $9.0 million was outstanding, respectively, on the line of credit. The Company also has notes payable of $350,000 each to PPD and Axys, another significant stockholder of the Company. Each of these $350,000 notes carries an interest rate of 8%. A total of $198,000 in accrued interest payable at September 30, 2000 related to all the notes payable is included in other current liabilities on the balance sheet.
F-34
5. COMMITMENTS
Licensing Agreements
The Company has entered into product/technology licensing agreements which require the payment of royalties on sales of certain products. As of September 30, 2000 no products have been sold which utilized the licensed technology and, accordingly, no royalties had been paid under these agreements.
Leases
The Company leases its office and research facilities under operating lease agreements which expire in 2001. The facilities leases are subject to terms which include deferred payment terms, annual escalation provisions and require the Company to pay operating costs on a pro rata basis to cover various maintenance, property tax and administrative expenses of the lessor. The Company also has an equipment operating lease which expires in 2004.
Annual future minimum payments for the obligations under operating leases are as follows as of September 30, 2000 (in thousands):
| Year ending December 31, |
Third Party |
Related Party |
Total Operating Leases |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 2000 | $ | 145 | $ | 67 | $ | 212 | |||
| 2001 | 579 | 84 | 663 | ||||||
| 2002 | — | 38 | 38 | ||||||
| 2003 | — | 38 | 38 | ||||||
| Thereafter | — | 153 | 153 | ||||||
| Total minimum lease payments | $ | 1,104 | |||||||
Rent expense on the facilities and equipment during the period from January 25, 1999 (inception) through December 31, 1999, the period from January 25, 1999 (inception) through September 30, 1999, and the nine months ended September 30, 2000 was $434,000, $292,000 and $681,000, respectively.
6. STOCKHOLDERS' EQUITY
Convertible Series A Preferred Stock
The Series A Preferred stock is convertible, at the option of the holder, into shares of the Company's common stock on a one-to-one basis, subject to certain antidilution adjustments. Annual noncumulative dividends for Series A Preferred stock are payable when and if declared by the Board of Directors. Dividends shall not be payable on the common shares unless a dividend is paid on each share of Series A Preferred stock equal to or greater than the amount of the common stock dividend.
The Series A Preferred stock will convert automatically upon the closing of an underwritten public offering of the Company's common stock.
F-35
In the event of a liquidation, dissolution or winding up of the Company, either voluntary or involuntary, the holders of the preferred shares are entitled to receive, before any amount shall be paid to holders of common shares, an amount equal to $1.00 per share for the Series A Preferred, plus any declared but unpaid dividends.
In all matters, with the exception of the election of directors, the holder of each share of Series A Preferred stock shall have the right to one vote for each share of common stock into which such shares of Series A Preferred stock could then be converted. Holders of the Series A Preferred stock voting as a separate class are entitled to elect four directors. All remaining directors are elected by the common stockholders and Series A Preferred stockholders voting together as one class.
Stock Option Plan
In February 1999, the Company established the 1999 Stock Option Plan (the "1999 Plan") for eligible employees, officers and directors. The 1999 Plan provides for the issuance of up to 1,775,000 shares of common stock under incentive and nonqualified stock options. Terms of the stock option agreements, including vesting requirements, are determined by the Board of Directors, subject to the provisions of the 1999 Plan.
Options granted by the Company generally vest over four years and are exercisable from the date of grant for a period of ten years. The exercise price of the incentive stock options must equal at least the fair value on the date of grant or 110% of the fair value in the case of any person possessing 10% combined voting power for all classes of stock of the Company. The exercise price of nonstatutory stock options may be less than 100% of the fair value of the stock on the date of grant.
The following table summarizes stock option activity under the Plan:
| |
Number of Shares |
Exercise Price |
||||
|---|---|---|---|---|---|---|
| Granted | 1,837,500 | $ | 2.00 | |||
| Canceled | (481,500 | ) | $ | 2.00 | ||
| Balance at December 31, 1999 | 1,356,000 | $ | 2.00 | |||
| Granted | 290,200 | $ | 2.00 | |||
| Canceled | (294,200 | ) | $ | 2.00 | ||
| Exercised | (25,000 | ) | $ | 2.00 | ||
| Balance at September 30, 2000 | 1,327,000 | $ | 2.00 | |||
The weighted-average remaining contractual life of options outstanding under the 1999 Plan is approximately nine years. At September 30, 2000, 395,980 options issued under the 1999 Plan were exercisable at $2.00 per share. At September 30, 2000, options for a total of 448,000 shares were available for future grant under the Plan.
Adjusted pro forma information regarding net loss is required by SFAS No. 123, and has been determined as if the Company had accounted for its employee stock options under the fair value
F-36
method of that Statement. The fair value for these options was estimated at the date of grant using the "minimum value" method for option pricing with the following weighted average assumptions: risk-free interest rate of 6.5%; dividend yield of 0%; and a weighted-average expected life of the options of three to five years. The effect of applying the minimum value method of SFAS No. 123 to options granted to employees in 1999 did not result in a pro forma net loss that was significantly different from historical amounts reported for the period from January 25, 1999 (inception) through December 31, 1999.
7. RELATED PARTY TRANSACTIONS
The Company subleases certain office facilities from related parties. The expense related to these leases for the period from January 25, 1999 (inception) to December 31, 1999, the period from January 25, 1999 (inception) to September 30, 1999, and the nine months ended September 30, 2000 was $371,000, $292,000, and $681,000, respectively. On July 20, 2000, the Company's majority stockholder assigned the lease for its La Jolla, California facility to an unrelated third party. The lease terms were not changed as a result of this assignment. During the period from January 25, 1999 (inception) to December 31, 1999, the Company recognized $800,000 of expense for services provided by employees of its principal stockholders and purchased fixed assets totaling $213,000. In addition, in accordance with a distributor agreement dated February 1, 2000, certain of the Company's products and services are exclusively marketed and sold to PPD for a commission. During the period from January 25, 1999 (inception) to December 31, 1999 and the nine months ended September 30, 2000, the Company recognized commissions for services provided by PPD employees totaling $380,000 and $508,000, respectively. Such amounts have been recorded as an offset to revenues. Management believes the services purchased and assets acquired were obtained at fair value. At December 31, 1999 and September 30, 2000, $154,000 and $421,000 was due from the minority stockholder related to sales recognized in 1999 and 2000, respectively.
8. INCOME TAXES
Significant components of the Company's deferred tax assets as of December 31, 1999 are shown below. A valuation allowance of $2,477,000 has been recognized to offset the deferred tax assets as realization of such assets at December 31, 1999 is uncertain.
| |
December 31, 1999 |
|||||
|---|---|---|---|---|---|---|
| Deferred tax assets (in thousands): | ||||||
| Net operating loss carryforwards | $ | 2,074 | ||||
| Research and development credits | 196 | |||||
| Other | 207 | |||||
| Total deferred tax assets | 2,477 | |||||
| Less valuation allowance | (2,477 | ) | ||||
| Net deferred tax assets | $ | — | ||||
F-37
At December 31, 1999, the Company had federal and California tax net operating loss carryforwards of approximately $5.5 million and $2.8 million, respectively. The federal and state tax loss carryforwards will begin expiring in 2019 and 2004, respectively, unless previously utilized. The Company also has federal and California research and development tax credit carryforwards of $162,000 and $53,000 respectively, which will begin expiring in 2014 unless previously utilized.
Pursuant to Internal Revenue Code Section 382, annual use of the tax net operating carryforwards may be limited if a cumulative change in ownership of greater than 50% occurs within any three-year period. However, the Company does not believe such limitations will have a material impact upon the utilization of these carryforwards.
9. SUBSEQUENT EVENT
In December, 2000 all of the Company's outstanding stock was acquired by DNA Sciences, Inc.
F-38
DNA Sciences, Inc.
(a development stage company)
UNAUDITED PRO FORMA COMBINED CONDENSED FINANCIAL STATEMENTS
The following Unaudited Pro Forma Combined Condensed Financial Statements for DNA Sciences, Inc. ("the Company") have been prepared to illustrate the effects of the acquisition of PPGx Inc. ("PPGx"). In exchange for all the outstanding equity of PPGx, PPGx stockholders received approximately 2,963,000 shares of the Company's common and Series D redeemable convertible preferred shares.
The purchase price of PPGx was estimated at approximately $36.5 million based upon the estimated fair value of the Company's common and Series D redeemable preferred stock issued, the fair value of approximately 306,000 options to purchase common stock issued in exchange for options held by PPGx employees and $500,000 of estimated direct acquisition costs. The excess of the purchase price over the fair value of net assets acquired has been allocated to goodwill and identified intangible assets. The allocation is preliminary and subject to adjustment upon the completion of an independent valuation. The goodwill is expected to be amortized over 5 years and the intangible assets over 2 to 5 years.
Pro forma amounts have been derived by applying pro forma adjustments to the historical financial information of the Company and PPGx.
The Unaudited Pro Forma Combined Condensed Statements of Operations for the year ended December 31, 1999 and the nine months ended September 30, 2000, combine the Company's results of operations with PPGx's results of operations for such periods and have been prepared as if the PPGx acquisition had occurred on January 1, 1999. The Unaudited Pro Forma Combined Condensed Balance Sheet combines the Company's balance sheet at September 30, 2000 with PPGx's balance sheet at September 30, 2000 and has been prepared as if the PPGx acquisition occurred on September 30, 2000.
The unaudited pro forma financial information should not be considered indicative of actual results that would have been achieved had the acquisition been consummated on the dates indicated and does not purport to indicate balance sheet data or results of operations of any future date or any future period. In addition, the financial information does not include any costs related to the future integration of PPGx which have not been determined. The unaudited pro forma financial information should be read in conjunction with the historical financial statements of the Company and PPGx and the related notes thereto included elsewhere in this prospectus.
F-39
DNA Sciences, Inc.
UNAUDITED PRO FORMA COMBINED CONDENSED BALANCE SHEET
September 30, 2000
(in thousands)
| |
Historical |
|
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
DNA Sciences |
PPGx |
Pro Forma Adjustments |
Pro Forma Combined |
|||||||||||
Assets |
|||||||||||||||
Current assets: |
|||||||||||||||
| Cash and cash equivalents | $ | 40,882 | $ | 352 | $ | — | $ | 41,234 | |||||||
| Other current assets | 653 | 558 | — | 1,211 | |||||||||||
| Total current assets | 41,535 | 910 | — | 42,445 | |||||||||||
| Property and equipment, net | 18,079 | 2,283 | 20,362 | ||||||||||||
| DNA samples and intangible assets | — | — | 33,740 | (a) | 33,740 | ||||||||||
| Other assets | 1,839 | — | — | 1,839 | |||||||||||
| Total assets | $ | 61,453 | $ | 3,193 | $ | 33,740 | $ | 98,386 | |||||||
Liabilities and stockholders' equity (net capital deficiency) |
|||||||||||||||
Current liabilities: |
|||||||||||||||
| Accounts payable | $ | 3,852 | $ | 412 | $ | — | $ | 4,264 | |||||||
| Accrued liabilities | 608 | 862 | 500 | (b) | 1,970 | ||||||||||
| Due to related parties | 2,970 | 874 | — | 3,844 | |||||||||||
| Notes payable | — | 9,000 | (9,000) | (g) | — | ||||||||||
| Equipment financing—current portion | 2,957 | — | — | 2,957 | |||||||||||
| Total current liabilities | 10,387 | 11,148 | (8,500 | ) | 13,035 | ||||||||||
| Equipment financing—long term portion | 5,520 | — | — | 5,520 | |||||||||||
| Redeemable convertible preferred stock | 69,809 | — | 34,011 | (d) | 103,820 | ||||||||||
| Stockholders' equity (net capital deficiency): | |||||||||||||||
| Convertible preferred stock | — | 10 | (10) | (c) | — | ||||||||||
| Common stock | 4 | — | — | 4 | |||||||||||
| Additional paid-in capital | 22,824 | 4,656 | (4,656) | (c) | 25,247 | ||||||||||
| 2,423 | (d) | ||||||||||||||
| Notes receivable from stockholders | (244 | ) | — | — | (244 | ) | |||||||||
| Accumulated other comprehensive income | — | (35 | ) | 35 | (c) | — | |||||||||
| Deferred stock compensation | (15,205 | ) | — | (449) | (a) | (15,654 | ) | ||||||||
| Accumulated deficit | (31,642 | ) | (12,586 | ) | 12,586 | (c) | (33,342 | ) | |||||||
| (1,700) | (a) | ||||||||||||||
| Total stockholders' equity (net capital deficiency) | (24,263 | ) | (7,955 | ) | 8,229 | (23,989 | ) | ||||||||
| Total liabilities and stockholders' equity (net capital deficiency) | $ | 61,453 | $ | 3,193 | $ | 33,740 | $ | 98,386 | |||||||
See accompanying notes.
F-40
DNA Sciences, Inc.
UNAUDITED PRO FORMA COMBINED CONDENSED
STATEMENT OF OPERATIONS
For the period ended December 31, 1999
(in
thousands, except share and per share data)
| |
Historical |
|
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Pro Forma Adjustments |
Pro Forma Combined |
|||||||||||||
| |
DNA |
PPGx |
|||||||||||||
| Revenues | $ | 79 | $ | 887 | $ | — | $ | 966 | |||||||
| Operating expenses: | |||||||||||||||
| Cost of revenues | — | 1,030 | — | 1,030 | |||||||||||
| Research and development | 4,901 | 2,121 | 7,022 | ||||||||||||
| General and administrative | 2,700 | 3,818 | — | 6,518 | |||||||||||
| Stock-based compensation | 418 | — | 112 | (f) | 530 | ||||||||||
| Amortization of samples, goodwill and other acquisition related intangibles | — | — | 9,158 | (e) | 9,158 | ||||||||||
| Total operating expenses | 8,019 | 6,969 | 9,270 | 24,258 | |||||||||||
| Loss from operations | (7,940 | ) | (6,082 | ) | (9,270 | ) | (23,292 | ) | |||||||
| Interest income (expense), net | 103 | (83 | ) | — | 20 | ||||||||||
| Net loss | $ | (7,837 | ) | $ | (6,165 | ) | $ | (9,270 | ) | $ | (23,272 | ) | |||
| Basic and diluted net loss per share | $ | (16.01 | ) | $ | (46.95 | ) | |||||||||
| Weighted average shares of common stock outstanding used in computing basic and diluted net loss per share |
489,382 | 495,686 | |||||||||||||
See accompanying notes.
F-41
DNA Sciences, Inc.
UNAUDITED PRO FORMA COMBINED CONDENSED
STATEMENT OF OPERATIONS
For the nine months ended September 30, 2000
(in thousands, except share and per share data)
| |
Historical |
|
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Pro Forma Adjustments |
Pro Forma Combined |
|||||||||||||
| |
DNA |
PPGx |
|||||||||||||
| Revenues | $ | 500 | $ | 1,172 | — | $ | 1,672 | ||||||||
| Operating expenses: | |||||||||||||||
| Cost of revenues | — | 1,243 | — | 1,243 | |||||||||||
| Research and development | 14,602 | 2,506 | 17,108 | ||||||||||||
| General and administrative | 4,652 | 3,457 | — | 8,109 | |||||||||||
| Stock-based compensation | 5,985 | — | 84 | (f) | 6,069 | ||||||||||
| Amortization of samples, goodwill and other acquisition related intangibles | — | — | 6,869 | (e) | 6,869 | ||||||||||
| Total operating expenses | 25,239 | 7,206 | 6,953 | 39,398 | |||||||||||
| Loss from operations | (24,739 | ) | (6,034 | ) | (6,953 | ) | (37,726 | ) | |||||||
| Interest income (expense), net | 1,282 | (387 | ) | — | 895 | ||||||||||
| Net loss | $ | (23,457 | ) | $ | (6,421 | ) | $ | (6,953 | ) | $ | (36,831 | ) | |||
| Basic and diluted net loss per share | $ | (16.59 | ) | $ | (25.93 | ) | |||||||||
| Weighted average shares of common stock outstanding used in computing basic and diluted net loss per share | 1,413,990 | 1,420,294 | |||||||||||||
See accompanying notes.
F-42
DNA Sciences, Inc.
NOTES TO UNAUDITED PRO FORMA COMBINED CONDENSED FINANCIAL STATEMENTS
September 30, 2000
1. BASIS OF PRESENTATION
On December 22, 2000, DNA Sciences, Inc. (the "Company") acquired all of the outstanding capital stock and stock options of PPGx, Inc. ("PPGx"), in a transaction accounted for as a purchase business combination.
The Unaudited Pro Forma Combined Condensed Financial Statements have been prepared on the basis of assumptions described in the following notes and include assumptions relating to the allocation of the consideration paid for the assets and liabilities of PPGx based on preliminary estimates of fair value. The actual allocation of the consideration to be paid may differ from those assumptions reflected in the Unaudited Pro Forma Combined Condensed Financial Statements after final valuation and other procedures to be performed have been completed, including the receipt of an independent valuation report. The accompanying Unaudited Pro Forma Combined Condensed Statement of Operations (the "Pro Forma Statement of Operations") for the year ended December 31, 1999 and the nine months ended September 30, 2000 gives effect to the PPGx acquisition, as if it had occurred on January 1, 1999. The Unaudited Pro Forma Combined Condensed Balance Sheet (the "Pro Forma Balance Sheet") gives effect to the acquisition of PPGx as if the acquisition had occurred on September 30, 2000. The Pro Forma Statement of Operations and Pro Forma Balance Sheet and accompanying notes (the "Pro Forma Financial Information") should be read in conjunction with, and are qualified by reference to, the historical financial statements of the Company and PPGx and the related notes thereto.
The pro forma information should not be considered indicative of actual results that would have been achieved had the acquisition been consummated on the dates indicated and does not purport to indicate balance sheet data or results of operations as of any future date or any future period.
F-43
2. PRO FORMA ASSUMPTIONS
The following represents the preliminary allocation of the purchase price to the acquired assets and assumed liabilities of PPGx at September 30, 2000 and is included for illustrative pro forma purposes only.
| Calculation of purchase price (in thousands): | |||||
| Fair value of common shares and Series D preferred shares issued | $ | 34,084 | |||
| Fair value of stock options issued | 2,351 | ||||
| Less: Intrinsic value of unvested options to be recorded as deferred stock compensation | (449 | ) | |||
| Estimated transaction costs | 500 | ||||
| Total purchase price | $ | 36,486 | |||
| Preliminary allocation to (in thousands): | |||||
| Fair value of net tangible assets assumed | $ | 1,046 | |||
| In-process research and development | 1,700 | ||||
| Assembled workforce | 1,100 | ||||
| Developed and core technology | 6,500 | ||||
| DNA samples | 15,600 | ||||
| Goodwill and other intangibles | 10,540 | ||||
| $ | 36,486 | ||||
The actual allocation of the purchase price will depend upon the composition of PPGx net assets on the closing date of the agreement and the Company's valuation of the fair value of the net assets as of that date.
Intangible assets are expected to be amortized over the following periods:
| Intangible Asset |
Expected Period of Benefit |
|
|---|---|---|
| Goodwill and other intangibles | 5 years | |
| Assembled workforce | 2 years | |
| Developed and core technology | 5 years | |
| DNA samples | 3 years |
3. PRO FORMA ADJUSTMENTS
The pro forma financial information reflects the following adjustments:
F-44
4. PRO FORMA NET LOSS PER SHARE
Pro forma basic and diluted loss per share are calculated by dividing pro forma net loss by the shares used to calculate loss per share in the historical period. The effect of the common shares and options that were exchanged or assumed in connection with the acquisition of PPGx. and shares subject to repurchase are excluded from the calculation of diluted loss per share in a loss period as the effect would be antidilutive.
F-45
[GRAPHIC -- BACKGROUND MAP]
Shares
[LOGO]
DNA Sciences, Inc.
Common Stock
PROSPECTUS
, 2001
LEHMAN BROTHERS
CIBC WORLD MARKETS
DAIN RAUSCHER WESSELS
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table lists the costs and expenses, other than underwriting discounts and commissions, which we expect to incur in connection with the issuance and distribution of the securities being registered. Except for the SEC registration fee, the NASD filing fee, and the Nasdaq National Market fee, the amounts listed below are estimates:
| SEC Registration Fee | $ | 31,250 | ||
| NASD filing fee | $ | 13,000 | ||
| Nasdaq National Market Listing application fee | $ | 95,000 | ||
| Legal fees and expenses | $ | 700,000 | ||
| Blue sky fees and expenses | $ | 5,000 | ||
| Accounting fees and expenses | $ | 400,000 | ||
| Printing and engraving expenses | $ | 250,000 | ||
| Transfer agent and registrar fees | $ | 10,000 | ||
| Miscellaneous expenses | $ | 95,750 | ||
Total |
$ |
1,600,000 |
||
Item 14. Indemnification of Directors and Officers.
As permitted by Delaware law, our amended and restated certificate of incorporation provides that no director of ours will be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director, except for liability:
Our amended and rested certificate of incorporation further provides that we must indemnify our directors and officers and may indemnify or employees and agents to the fullest extent permitted by Delaware law. We believe that indemnification under our amended and restated certificate of incorporation covers negligence and gross negligence on the part of indemnified parties.
We intend to enter into indemnification agreements with each of our directors and officers. These agreements, among other things, will require us to indemnify each director and officer for certain expenses including attorneys' fees, judgments, fines and settlement amounts incurred by any such person in any action or proceeding, including any action by or in the right of DNA Sciences, Inc. arising out of the person's services as our director or officer, any subsidiary of ours or any other company or enterprise to which the person provides services at our request.
The underwriting agreement (Exhibit 1.1) will provide for indemnification by the underwriters of DNA Sciences, Inc., our directors, our officers who sign the registration statement, and our controlling persons for some liability arising under the Securities Act.
II-1
Item 15. Recent Sales of Unregistered Securities.
The following sets forth information regarding securities sold by the Registrant since inception:
1. In March 1999 and January 2000, we issued an aggregate of 8,283,000 shares of Series A preferred stock to 14 accredited investors at $2.00 per share, of which 2,250,000 shares were sold to an entity related to one of our directors.
2. In March 2000, we issued 7,099,006 shares of Series B preferred stock to 19 accredited investors at $7.50 per share, of which 4,333,336 shares were sold to nine of our executive officers and/or directors (and related entities).
3. In April 2000, we issued a warrant to purchase 200,000 shares of Series B preferred stock to an accredited investor, at an exercise price of $7.50 per share.
4. In August 2000, we issued a warrant to purchase 10,000 shares of common stock to an accredited investor, at an exercise price of $7.50 per share.
5. In October 2000, we issued two warrants to purchase an aggregate of 54,000 shares of Series B preferred stock at an exercise price of $7.50 per share to a consultant.
6. In December 2000, we issued 2,683,343 shares of Series C preferred stock to 20 accredited investors at $10.15 per share, of which 394,087 shares were sold to three of our executive officers and/or directors (and related entities).
7. In December 2000, we issued 438,565 shares of Series C-1 preferred stock to one corporate accredited investor at $10.15 per share.
8. In December 2000, we issued 2,597,100 shares of Series D preferred stock at a fair value of $14.37 per share to two accredited investors and 6,306 shares of common stock at a fair value of $2.05 per share to two accredited investors and two non-accredited investors. These shares were issued in connection with our acquisition of PPGx, Inc.
9. Between January 1999 and February 2000, we issued and sold an aggregate of 4,008,000 shares of common stock at prices ranging from $0.0005 to $0.20 per share to employees and consultants pursuant to restricted stock agreements.
10. Between July 1998 and December 2000, we granted options to purchase an aggregate of 5,679,824 shares of common stock at exercise prices ranging from $0.20 to $2.05 per share with a weighted exercise price of $0.93 per share.
The sales and issuances of common stock made pursuant to the exercise of stock options under the 2000 Equity Incentive Plan and restricted stock purchase agremeents to our officers, directors, employees and consultants as described in paragraphs (9)above were made in reliance on Rule 701 promulgated under the Securities Act. With respect to the grant of stock options in paragraph (10), an exemption from registration was unnecessary in that none of the transactions involved a "sale" of securities as term is used in Section 2(3) of Securities Act.
The sales and issuances of securities in the transactions described in paragraphs 1 through 8 above were made in reliance on Section 4(2) of the Securities Act or Rule 506 of Regulation D promulgated under the Securities Act. These sales were made without general solicitation or advertising. Each purchaser was a sophisticated investor with access to all relevant information necessary to evaluate the investment and represented to the us that the shares were being acquired for investment and not with a view to distribution.
II-2
Item 16. Exhibits and Financial Statement Schedules.
| Exhibit Number |
Description |
|
|---|---|---|
| 1.1* | Underwriting Agreement | |
| 3.1* | Amended and Restated Certificate of Incorporation of the Registrant | |
| 3.2* | Form of Amended and Restated Certificate of Incorporation of the Registrant, to be effective upon the closing of the offering | |
| 3.3* | Amended and Restated Bylaws of the Registrant | |
| 4.1* | Specimen Common Stock Certificate | |
| 4.2 | Form of Warrant to Purchase Series B Preferred Stock | |
| 5.1* | Opinion of Cooley Godward LLP | |
| 10.1 | Amended and Restated Investor Rights Agreement, dated December 22, 2000 between the Registrant and the investors listed therein | |
| 10.2 | 2001 Equity Incentive Plan | |
| 10.3 | 2001 Employee Stock Purchase Plan | |
| 10.4 | 2001 Non-Employee Directors' Plan | |
| 10.5*† | Research, Development and Commercialization Agreement between the Registrant and Amersham Pharmacia Biotech, dated December 29, 2000 | |
| 10.6*† | Collaboration Agreement between the Registrant and Healtheon/WebMD, as amended, dated March 16, 2000 | |
| 10.7*† | Master Agreement between the Registrant and the University of Utah, dated December 29, 2000 | |
| 10.8*† | Amended and Restated Distributor Agreement between PPGx, Inc. and Pharmaceutical Product Development, Inc., dated December 22, 2000 | |
| 10.9 | Security Agreement and Promissory Note between the Registrant and Dr. Hugh Rienhoff, dated April 12, 1999 | |
| 10.10 | Security Agreement and Promissory Note between the Registrant and Dr. Hugh Rienhoff, dated January 24, 2000 | |
| 10.11 | Security Agreement and Promissory Note between the Registrant and Dr. Gregory Went, dated December 22, 1999 | |
| 10.12 | Security Agreement and Promissory Note between the Registrant and Dr. Gregory Went, dated November 30, 1999 | |
| 10.13 | Security Agreement and Promissory Note between the Registrant and Steven Lehrer, dated December 30, 1999 | |
| 10.14 | Security Agreement and Promissory Note between the Registrant and Steven Lehrer, dated December 30, 1999 | |
| 10.15 | Pledge Agreement and Full Recourse Secured Promissory Note between the Registrant and Dr. Ray White, dated July 27, 2000 | |
| 10.16 | Employment Agreement between the Registrant and Dr. Hugh Rienhoff dated March 9, 1999 | |
| 10.17 | Agreement and Plan of Merger and Reorganization among the Registrant, PIPO Acquisition Corp. and PPGx, Inc., dated December 17, 2000, as amended | |
| 23.1 | Consent of Ernst & Young, independent public accountants | |
| 23.2 | Consent of Ernst & Young, independent public accountants | |
| 24.1 | Power of Attorney (see signature page) |
II-3
All schedules for which provision is made in the applicable accounting regulations of the Securities and Exchange Commission are not required under the related instructions or are inapplicable, and therefore have been omitted.
Item 17. Undertakings.
We hereby undertake to provide to the underwriter at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of DNA Sciences pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by a director, officer or controlling person of DNA Sciences in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
We hereby undertake that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by us pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act will be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus will be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time will be deemed to be the initial bona fide offering thereof.
II-4
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Fremont, State of California, on this 5th day of January, 2001.
| DNA SCIENCES, INC. | |||
By: |
|||
| /s/ HUGH RIENHOFF Hugh Y. Rienhoff, Jr., M.D. Chairman and Chief Executive Officer |
|||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints, jointly and severally, Hugh Y. Rienhoff, Jr., M.D., and Susan Berland, and each or any one of them, his or her true and lawful attorneys-in-fact and agents for the undersigned and for each of them, each with full power of substitution and resubstitution, for him and her and in his and her name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and any registration statement related to this offering contemplated by this registration statement that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates stated.
| Signatures |
Title |
Date |
||
|---|---|---|---|---|
/s/ HUGH RIENHOFF Hugh Y. Rienhoff, Jr. M.D. |
Chairman and Chief Excutive Officer (Principal Executive Officer) | January 5, 2001 | ||
/s/ SUSAN BERLAND Susan Berland |
Chief Financial Officer (Principal Financial and Accounting Officer) |
January 5, 2001 |
||
/s/ BRIAN ATWOOD Brian Atwood |
Director |
Janaury 5, 2001 |
||
II-5
/s/ WALTER BURLOCK Walter Burlock |
Director |
January 5, 2001 |
||
/s/ JAMES CLARK James Clark, Ph.D. |
Director |
January 5, 2001 |
||
/s/ JAMES WATSON James Watson, Ph.D. |
Director |
January 5, 2001 |
||
/s/ GREGORY WENT Gregory T. Went, Ph.D. |
Director |
January 5, 2001 |
II-6
| Exhibit Number |
Description |
|
|---|---|---|
| 1.1* | Underwriting Agreement | |
| 3.1* | Amended and Restated Certificate of Incorporation of the Registrant | |
| 3.2* | Form of Amended and Restated Certificate of Incorporation of the Registrant, to be effective upon the closing of the offering | |
| 3.3* | Amended and Restated Bylaws of the Registrant | |
| 4.1* | Specimen Common Stock Certificate | |
| 4.2 | Form of Warrant to Purchase Series B Preferred Stock | |
| 5.1* | Opinion of Cooley Godward LLP | |
| 10.1 | Amended and Restated Investor Rights Agreement, dated December 22, 2000 between the Registrant and the investors listed therein | |
| 10.2 | 2001 Equity Incentive Plan | |
| 10.3 | 2001 Employee Stock Purchase Plan | |
| 10.4 | 2001 Non-Employee Directors' Plan | |
| 10.5*† | Research, Development and Commercialization Agreement between the Registrant and Amersham Pharmacia Biotech, dated December 29, 2000 | |
| 10.6*† | Collaboration Agreement between the Registrant and Healtheon/WebMD, as amended, dated March 16, 2000 | |
| 10.7*† | Master Agreement between the Registrant and the University of Utah, dated December 29, 2000 | |
| 10.8*† | Amended and Restated Distributor Agreement between PPGx, Inc. and Pharmaceutical Product Development, Inc., dated December 22, 2000 | |
| 10.9 | Security Agreement and Promissory Note between the Registrant and Dr. Hugh Rienhoff, dated April 12, 1999 | |
| 10.10 | Security Agreement and Promissory Note between the Registrant and Dr. Hugh Rienhoff, dated January 24, 2000 | |
| 10.11 | Security Agreement and Promissory Note between the Registrant and Dr. Gregory Went, dated December 22, 1999 | |
| 10.12 | Security Agreement and Promissory Note between the Registrant and Dr. Gregory Went, dated November 30, 1999 | |
| 10.13 | Security Agreement and Promissory Note between the Registrant and Steven Lehrer, dated December 30, 1999 | |
| 10.14 | Security Agreement and Promissory Note between the Registrant and Steven Lehrer, dated December 30, 1999 | |
| 10.15 | Pledge Agreement and Full Recourse Secured Promissory Note between the Registrant and Dr. Ray White, dated July 27, 2000 | |
| 10.16 | Employment Agreement between the Registrant and Dr. Hugh Rienhoff dated March 9, 1999 | |
| 10.17 | Agreement and Plan of Merger and Reorganization among the Registrant, PIPO Acquisition Corp. and PPGx, Inc., dated December 17, 2000, as amended | |
| 23.1 | Consent of Ernst & Young, independent public accountants | |
| 23.2 | Consent of Ernst & Young, independent public accountants | |
| 24.1 | Power of Attorney (see signature page) |
|
|