|
|
|
|
|
Previous: AMERICAN MEDICAL LABORATORIES INC, S-1, EX-27.1, 2000-09-29 |
Next: APPLIED EPI INC, S-1, EX-1.1, 2000-09-29 |
Registration No. 333-
| Minnesota | 3559 | 41-1569588 | ||
|
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
4900 Constellation Drive
David G. Reamer
Copies to:
|
John R. Houston, Esq. Robins, Kaplan, Miller & Ciresi L.L.P. 2800 LaSalle Plaza 800 LaSalle Avenue Minneapolis, Minnesota 55402 (612) 349-8500 |
Christopher D. Lueking, Esq. Latham & Watkins 233 South Wacker Drive Sears Tower, Suite 5800 Chicago, Illinois 60606 (312) 876-7700 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. [ ]
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. [ ]
CALCULATION OF REGISTRATION FEE
| Proposed Maximum | ||||
| Title of Each Class of | Aggregate Offering | Amount of | ||
| Securities to be Registered | Price(1)(2) | Registration Fee | ||
| Common Stock, $0.01 par value | $97,095,978 | $25,633 | ||
| (1) | Estimated pursuant to Rule 457(o) of the Securities Act of 1933 solely for the purpose of computing the amount of the registration fee. |
| (2) | Includes shares that the Underwriters have the option to purchase from us solely to cover over-allotments, if any. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission acting pursuant to said Section 8(a), may determine.
| The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting offers to buy securities in any state where the offer or sale is not permitted. |
SUBJECT TO COMPLETION, DATED , 2000
PROSPECTUS
Shares

Common Stock
Applied Epi, Inc. is offering shares of its common stock. This is our initial public offering and no public market currently exists for our shares. We expect the initial public offering price to be between $ and $ per share.
We have applied for quotation of our common stock on the Nasdaq National Market under the symbol “APEP.”
Investing in our common stock involves risks that are described in the “Risk Factors” section beginning on page 6 of this prospectus.
| Per Share | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discount | $ | $ | ||||||
| Proceeds, before expenses, to Applied Epi | $ | $ | ||||||
The underwriters may also purchase up to an additional shares from Applied Epi at the public offering price, less the underwriting discount, within 30 days from the date of this prospectus to cover over-allotments.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The shares will be ready for delivery on or about , 2000.
Joint Book-Running Managers
| Donaldson, Lufkin & Jenrette | Merrill Lynch & Co. |
Wit SoundView
The date of this prospectus is , 2000.
Inside Gatefold Pages:
The first page of the gatefold includes the following quotations, “Applied Epi’s equipment and services are used to produce compound semiconductors that enable the fiber optic and wireless communications infrastructure” and “Enabling Communications.” We also include pictures of the multiwafer handling cluster tool, portions of the periodic table and six pictures representing various applications that incorporate compound semiconductors.
The second page of the gatefold includes the heading “Market” and the following text, “Compound semiconductors are core components of a wide variety of communications applications that address the growing demand for connectivity and information bandwidth.” Next to this quotation is a picture of two people accessing the Internet via a laptop personal computer.
Also included on this page is the heading “Technology” and the following text, “Most compound semiconductors are produced using epitaxial technologies. Their advanced physical properties provide high-performance fiber optic and wireless systems with:
| • | Higher operating speeds | |
| • | Optoelectronic properties | |
| • | Lower power consumption | |
| • | Reduced signal noise and distortion.” |
Next to this text is a picture of a portion of the periodic table and an epiwafer.
On the bottom of page two of the gatefold are the words “Connectivity” and “Bandwidth.”
Page three of the gatefold includes the heading, “Solutions” and the following text “Our equipment and related components address the critical epitaxy process for the manufacture of compound semiconductors.” We also include pictures of the GEN2000, the GENII and some of our performance products with the following text next to each applicable picture:
HIGH VOLUME PRODUCTION SYSTEMS
GEN2000™ Multi-Wafer System
| • | Low cost per wafer | |
| • | High production capacity and throughput | |
| • | Unique cluster tool wafer management system | |
| • | Flexible modular design |
LOW VOLUME PRODUCTION AND RESEARCH SYSTEMS
GENII™ Single-Wafer System
| • | Ideal for optoelectronic materials | |
| • | Flexible modular design |
PERFORMANCE PRODUCTS
SUMO® Cells
| • | High source material capacity | |
| • | High epilayer uniformity and reproducibility |
Valved Crackers
| • | High source material capacity | |
| • | Precision molecular beam control |
At the bottom of the third page is the word “Solutions.”
On the bottom right hand corner of each of these pages is also the Applied Epi logo.
Table of Contents
| Page | ||||
| Prospectus Summary | 1 | |||
| Risk Factors | 6 | |||
| Forward-Looking Statements | 15 | |||
| Use of Proceeds | 16 | |||
| Dividend Policy | 17 | |||
| S Corporation Status | 17 | |||
| Capitalization | 18 | |||
| Dilution | 19 | |||
| Selected Financial Data | 20 | |||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations | 22 | |||
| Business | 32 | |||
| Management | 48 | |||
| Related Party Transactions | 54 | |||
| Principal Shareholders | 56 | |||
| Description of Capital Stock | 57 | |||
| Shares Eligible for Future Sale | 60 | |||
| Underwriting | 62 | |||
| Legal Matters | 66 | |||
| Experts | 66 | |||
| Additional Information | 66 | |||
| Index to Consolidated Financial Statements | F-1 | |||
You should rely only on the information contained in this prospectus. Neither we nor the underwriters have authorized any person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We and the underwriters are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus is accurate as of the date on the front cover of this prospectus only. Our business, financial condition, results of operations and prospects may have changed since that date.
This summary highlights selected information described more fully elsewhere in this prospectus. This summary is not complete and does not contain all the information you should consider before investing in our common stock. You should read the entire prospectus carefully, including the risks set forth in “Risk Factors” and the financial statements and their related notes, before making an investment decision.
We are a leading provider of epitaxial equipment and related components used to produce compound semiconductors for the fiber optic and wireless markets, as well as for other consumer applications. Our product line includes epitaxial equipment and related components used in both high and low volume commercial production, and research and development. For 14 years, we have offered products and services designed to cost-effectively meet the increasingly demanding production requirements of the global high-performance communications infrastructure. Our customers use our equipment and components to manufacture compound semiconductors for a wide variety of communications applications, including fiber optic modules and subsystems, mobile phones, wireless networks and satellites. Other uses for compound semiconductors include several rapidly growing consumer applications, such as digital versatile disks (DVDs), low cost lighting and signage and global positioning systems.
The growing demand for information and connectivity is driving the continued rapid expansion of wireless and fiber optic networks, and the products that rely on these networks. In the past, communications equipment and products relied on silicon semiconductor technology to meet performance requirements. However, fiber optic and current generations of wireless networks, and their related products, require higher performance and greater functionality than silicon semiconductors can provide. As a result, compound semiconductors have emerged as a key enabling technology to meet these higher performance requirements.
Compound semiconductors are composed of two or more elemental materials usually consisting of a metal and a non-metal. The intrinsic physical properties of compound semiconductors enable electrons to move approximately five times faster than through silicon semiconductors, allowing these semiconductors to operate at significantly higher speeds. In addition, compound semiconductors have optoelectronic properties that enable them to emit light, a fundamental requirement of fiber optic applications and a function not achievable using silicon semiconductors. Other key advantages include lower power consumption and reduced signal distortion, which are critical to the performance of current generations of wireless technologies.
According to Strategies Unlimited, the compound semiconductor market was approximately $11 billion in 1999 and is expected to grow 24% to $13.6 billion in 2000. Communications applications that use compound semiconductors account for approximately 50% of this market. Driven by the anticipated high demand for fiber optic and wireless communications, this segment is expected to grow faster than the overall compound semiconductor market.
For the majority of compound semiconductors, epitaxy is the critical first step of the fabrication process, ultimately determining device functionality and performance. Epitaxy is the process of precisely depositing atomically thin crystal layers, or epilayers, of elemental materials onto a substrate. After the epilayers are grown on the substrate, it is known as an epiwafer. The unique performance characteristics of compound semiconductors are dependent on the chemical composition, number, and precise thickness of the epilayers. As a result, epitaxy is considered to be the highest value-added step
1
We have been an innovator in the compound semiconductor market since our inception, and believe that we offer the broadest product selection of epitaxial equipment and related components for the compound semiconductor industry. Our GEN2000 is the world’s first high volume production MBE system incorporating ultra high vacuum (UHV) cluster tool architecture, designed to provide our customers with increased productivity and lower cost of ownership. With its ability to process seven 6” wafers simultaneously, it is also currently the world’s largest capacity production MBE system. We shipped our first GEN2000 in the third quarter of 2000. We also provide a full line of low volume production and research MBE systems that are capable of performing in a wide variety of environments. Our most popular system in this class, the Modular GENII, has an installed base of over 200 systems, giving us what we believe is a leading share in the market for low volume production and research MBE systems.
We are also a leading supplier of key components to the MBE equipment market, including our proprietary effusion cell technology, control hardware and electronics, process control software, spare parts and consumables. We estimate that more than 70% of the world’s compound semiconductor manufacturers using MBE systems use our effusion cells, the most critical component for the successful operation of an MBE system.
Our goal is to become the leading supplier of integrated equipment and related process solutions to compound semiconductor manufacturers for use throughout their fabrication line. Key elements of our strategy are as follows:
| • | further penetrate the high volume MBE equipment markets; | |
| • | leverage our current technology and relationships to penetrate the MOCVD market; | |
| • | expand our product offering to offer complete fabrication line solutions; | |
| • | enhance our customer relationships, including opening a Process Integration Center; and | |
| • | extend our technology leadership. |
We have a diverse global customer base with no single customer accounting for more than 10% of our sales in 1998 or 1999. We sell our products to compound semiconductor manufacturers and epiwafer suppliers, as well as research and academic institutions. In 1999, our major commercial customers in the fiber optic market included Bandwidth9, Inc., JDS Uniphase Corporation, Lucent Technologies Inc., Mitsubishi Chemical Corporation and NEC Corporation, and in the wireless market included Agilent Technologies, Inc., IQE plc, Raytheon Company, RF Micro Devices, Inc. and Sanders, a Lockheed Martin Company. International sales represented approximately 32% of our sales in 1997, 35% in 1998, 40% in 1999 and 38% in the six months ended June 30, 2000.
2
Additional Information
We were incorporated in Minnesota in 1986 under the name Effusion Products, Inc. In 1987, we changed our name to Chorus Corporation and in July 2000, we changed our name to Applied Epi, Inc. Our executive offices are located at 4900 Constellation Drive, St. Paul, MN 55127, and our telephone number is (651) 482-0800. The address of our website is www.applied-epi.com. Information contained on our website is not part of this prospectus.
SUMO® and Uniblock® are our registered trademarks. Applied Epi™, GEN2000™, GEN200™, GENII™, GENIII™, Molly™ and certain other names not included in this prospectus are our trademarks. Registrations of the Applied Epi™ name and logo as well as other names used in our business are pending. Each trademark, trade name or service mark of any other company appearing in this prospectus belongs to its holder.
3
The Offering
| Common stock offered by Applied Epi | shares | |
| Common stock to be outstanding after this offering | shares | |
| Use of proceeds | For construction and development of a Process Integration Center; expansion of our facilities; distributions to our existing shareholders in connection with termination of S corporation status and to holders of performance units in connection with termination of our Employee Performance Stock Ownership Plan upon completion of this offering; and working capital, acquisitions and general corporate purposes. | |
| Proposed Nasdaq National Market symbol | APEP |
The number of shares of common stock to be outstanding after this offering is based on the number of shares outstanding as of September 25, 2000. This information:
| • | excludes an aggregate of 6,000,000 shares of our common stock currently reserved for issuance under our 1993 Stock Option Plan, of which 1,952,248 shares are subject to outstanding options at a weighted average exercise price of $4.92 per share; | |
| • | excludes an aggregate of 6,000,000 shares of our common stock currently reserved for issuance under our 2000 Stock Plan, of which 242,000 shares are subject to outstanding options at an exercise price of $12.50 per share; and | |
| • | excludes conversion of phantom stock units issued under our Employee Performance Stock Ownership Plan into 621,482 shares of our common stock and excludes an aggregate of 734,388 units available for future issuance under the plan. |
Unless otherwise indicated, all information in this prospectus gives effect to the four-for-one stock split to be effected prior to this offering and assumes that the underwriters will not exercise their over-allotment option.
4
Summary Financial Data
The tables below summarize our financial data set forth in more detail in the consolidated financial statements at the end of this prospectus. The pro forma as adjusted balance sheet data reflect the sale by us of shares of common stock offered by this prospectus at an assumed initial public offering price of $ per share, after deducting the estimated underwriting discounts and commissions and offering expenses payable by us.
| Six Months Ended | ||||||||||||||||||||||||||||
| Year Ended December 31, | June 30, | |||||||||||||||||||||||||||
| 1995 | 1996 | 1997 | 1998 | 1999 | 1999 | 2000 | ||||||||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||||||||||||||
| Statement of Operations Data: | ||||||||||||||||||||||||||||
| Sales | $ | 12,641 | $ | 12,088 | $ | 11,854 | $ | 15,533 | $ | 15,389 | $ | 6,849 | $ | 10,180 | ||||||||||||||
| Gross profit(1) | 6,080 | 5,378 | 5,674 | 6,847 | 6,810 | 2,964 | 4,943 | |||||||||||||||||||||
| Operating expenses(2) | 3,806 | 3,694 | 4,102 | 3,443 | 3,343 | 1,526 | 2,887 | |||||||||||||||||||||
| Stock-based compensation | — | — | — | — | — | — | 7,224 | |||||||||||||||||||||
| Income (loss) from operations | 2,274 | 1,684 | 1,572 | 3,404 | 3,466 | 1,438 | (5,168 | ) | ||||||||||||||||||||
| Income (loss) before income taxes | 2,487 | 1,753 | 1,530 | 5,070 | 5,092 | 3,064 | (5,261 | ) | ||||||||||||||||||||
| Income taxes | 1,081 | 302 | 213 | — | — | — | — | |||||||||||||||||||||
| Net income (loss) | $ | 1,406 | $ | 1,451 | $ | 1,317 | $ | 5,070 | $ | 5,092 | $ | 3,064 | $ | (5,261 | ) | |||||||||||||
| Historical net income (loss) | 5,092 | (5,261 | ) | |||||||||||||||||||||||||
| Pro forma provision for income taxes(3) | 1,616 | 517 | ||||||||||||||||||||||||||
| Pro forma net income (loss)(3) | 3,476 | (5,778 | ) | |||||||||||||||||||||||||
| Pro forma net income (loss) per common share (basic and diluted)(3) | $ | 0.16 | $ | (0.26 | ) | |||||||||||||||||||||||
| Pro forma weighted average common shares (basic and diluted)(3) | 22,065 | 22,065 | ||||||||||||||||||||||||||
| As of June 30, 2000 | ||||||||||||
| Pro Forma | ||||||||||||
| Actual | Pro Forma(4) | As Adjusted | ||||||||||
| (in thousands) | ||||||||||||
| Balance Sheet Data: | ||||||||||||
| Cash and cash equivalents | $ | 1,885 | $ | 1,885 | ||||||||
| Working capital | 4,063 | 4,502 | ||||||||||
| Total assets | 21,832 | 22,810 | ||||||||||
| Long-term debt, less current portion | 5,107 | 5,107 | ||||||||||
| Total shareholders’ equity | 8,999 | 9,977 | ||||||||||
| (1) | Excludes stock-based compensation charge of $84,029 for the six months ended June 30, 2000. |
| (2) | Excludes stock-based compensation charge of $7,140,084 for the six months ended June 30, 2000. |
| (3) | Applied Epi operated as an S corporation since 1997 and will terminate such status in connection with this offering. See Note 3 to Notes to Consolidated Financial Statements for information concerning the computation of pro forma net income (loss) and pro forma net income (loss) per share. |
| (4) | Reflects adjustments to account for the deferred tax asset described in “S Corporation Status.” See Note 3 of Notes to Consolidated Financial Statements. |
5
Your investment in our common stock involves a high degree of risk. You should carefully consider the risks described below and all of the information contained in this prospectus before deciding whether to purchase our common stock. These risks and uncertainties are not the only ones facing our company. Additional risks and uncertainties that we are unaware of or currently deem immaterial may also become important factors that may harm our business. The occurrence of any of the following events could harm our company or the value of your shares. If any of these events occur, the trading price of our shares could decline, and you may lose all or part of your investment.
Risks Related to the Compound Semiconductor Industry
| Slower growth in demand or lack of development for applications that use compound semiconductors would reduce sales of our products and services and lower our profitability. |
Because the demand for epitaxial equipment and related components and services depends on the demand for applications that use compound semiconductors, our success in selling our products and services depends on the continued growth in demand for existing applications, and on the successful development and commercialization of emerging applications, that incorporate compound semiconductors. The rate of this growth could slow as a result of production overcapacity resulting in market saturation or in connection with changes in technology, consumer preferences or declines in disposable income resulting from a general economic downturn. The timing, duration and severity of any future downturn cannot be predicted. In the medium term, we believe that the most significant potential for future growth in the demand for epitaxial equipment and related components and services will be from currently emerging applications that use compound semiconductors. The commercial markets for these applications are still in their formative stages. If these and other emerging applications using compound semiconductors do not develop, or if demand for current applications does not grow, we would have difficulties in selling our products and services and our sales and profitability would be harmed.
| If we do not respond quickly enough to rapid technological change and evolving standards in the compound semiconductor industry, we may have difficulties selling our products and services. |
The compound semiconductor industry is characterized by rapid technological change, evolving industry standards, changing customer requirements and frequent new product introductions. Commitments to developing any new epitaxial system must be made well in advance of sales, and technology and standards may change while we are developing new models, making our systems outdated or uncompetitive before their introduction. To remain competitive, we expect to incur significant capital expenditures and devote significant resources for research and development. We may be unable to successfully design or manufacture new products and may have difficulty penetrating new markets. Our future success will depend, to a significant extent, on our ability to keep pace with changes in the market and on our ability to enhance our current products and introduce new products. If we do not anticipate or respond adequately to technological developments or customer requirements, we could lose market share and our sales and profitability would be harmed.
Risks Related to Our Business
| The intense competition in our industry could reduce or eliminate the demand for our products. |
Competition in the market for epitaxial equipment and related components and services is intense and we face substantial competition from a number of companies, some of which have greater financial, marketing, manufacturing and technical resources than us. Our competitors could spend
6
| The failure of our high volume production MBE systems to achieve market acceptance or delays in our production MOCVD system development efforts could harm our future growth, sales and profitability. |
We are entering new product and technology areas with the introduction earlier this year of our first high volume production MBE systems, the GEN2000 and GEN200, and with our current development of production MOCVD equipment to expand our product line. Our prior experience in manufacturing and marketing, and our historical sales, have been derived from MBE components and low volume production and research MBE systems. Many of our competitors have significantly more experience in developing, manufacturing and marketing high volume production MBE and production MOCVD systems. Our future growth, sales and profitability will be harmed if our high volume production MBE systems do not gain market acceptance. Delays in development or our inability to successfully develop and commercialize a production MOCVD system could harm our future growth, sales and profitability. In addition, some of our customer contracts for high volume production MBE systems currently require, and contracts for MOCVD systems we develop may require, that the system meet predetermined performance criteria. Our failure to satisfy these criteria would harm our ability to gain market acceptance for these systems and harm our future growth, sales and profitability.
| We are currently dependent on MBE technology, and advances in alternative compound semiconductor manufacturing technologies may reduce our competitiveness and reduce or eliminate demand for our products. |
All of our current products and services are related to MBE technology, which is one of several competing technologies used in the compound semiconductor manufacturing process. Customers may prefer alternative manufacturing techniques, such as MOCVD, to produce compound semiconductors. In addition, customers may manufacture products that employ alternative compound semiconductor technologies, such as silicon germanium. Technologies such as these could offer a competitive alternative to some of the compound semiconductor devices currently manufactured with MBE technology. Compound semiconductors are also manufactured by ion implant technology, which is not an epitaxial technology. If any alternative compound semiconductor manufacturing technology develops further, gains wider market acceptance and reduces or eliminates the competitive advantages of MBE, we may have difficulty selling new MBE systems and related components and services and our sales and profitability would be harmed.
7
| Our sales and operating results may fluctuate in future periods, which could harm our share price. |
Our sales and operating results may vary significantly from quarter to quarter due to a number of factors particular to us and the compound semiconductor industry. Not all of these factors are in our control. These factors include:
| • | the volume and timing of orders for our products, particularly our epitaxial production systems, which have relatively high average selling prices; | |
| • | the timing of customer acceptance of our epitaxial systems. In most cases, we do not recognize revenue from our system contracts until the customer accepts the system. Customer acceptance may occur upon installation or in some cases, upon demonstrated system performance some time after installation; | |
| • | the timing of our announcement and introduction of new products and of similar announcements by our competitors; | |
| • | downturns in the market for our customers’ products; and | |
| • | regional economic conditions, particularly in Europe and the Asia-Pacific region, from which we derive significant sales. |
These factors and other unanticipated events may cause our operating results for future quarterly periods to be below the expectations of analysts and investors. A large portion of our operating expenses, including depreciation, salaries and certain manufacturing expenses, is currently fixed and expected to increase as we grow. If net sales do not meet our expectations in a particular quarter, our profitability would be harmed and our stock price may decline. These factors also cause our period-to-period comparisons of our results of operations to be less meaningful and they should not be relied upon as indicative of future operating results.
| We depend upon key personnel, the loss of whom would harm our competitiveness and future growth. |
Our success is dependent on the efforts of some of our key employees, particularly Paul E. Colombo, our founder and Chairman, and David G. Reamer, our President and Chief Executive Officer. These individuals would be very difficult to replace. If either of these individuals were to leave us, the loss of their services would harm the development and implementation of our long-term strategy, customer relationships, operations and growth. Neither of these individuals is bound to serve us for any specified term nor do we have non-compete agreements with them.
| Our inability to recruit and retain qualified employees could harm our competitiveness and future growth. |
It is important to our future success that we attract and retain qualified scientific, technical, operations, sales, and management personnel. The competition for attracting and retaining these employees is intense. Industry demand for skilled employees, particularly scientific and technical personnel with compound semiconductor experience, exceeds the number of skilled personnel available. In the future, we may experience difficulty in hiring and retaining highly skilled employees with appropriate qualifications. If we do not succeed in attracting new personnel and retaining current personnel, our business could be harmed by the loss of accumulated knowledge of our business and a reduction in our ability to design, develop, manufacture, and market new epitaxial equipment and related components and services to meet evolving industry standards.
8
| We have a lengthy sales cycle for our epitaxial systems and, in many cases, must invest a substantial amount of time and funds for which we may never receive orders. |
Sales of our epitaxial equipment will depend primarily upon the decision of a prospective customer to increase its manufacturing capacity, which involves a significant capital commitment by the customer. The sales process associated with the purchase of our systems is typically complex and lengthy and could exceed one year while customers evaluate and receive internal approvals for the purchase of these systems. During this period, we will likely spend funds to continue our marketing and sales efforts to sell our systems. These expenditures and efforts may not result in sales of new epitaxial equipment and reduce our profitability.
| We have a lengthy cycle from commencement of manufacture of our systems until recognition of revenue and must invest a substantial amount of time and funds for which we may never recognize revenue. |
Because we have lengthy manufacturing cycles, which can exceed six months for our current systems, we may assume forward purchasing risks by outsourcing the manufacture of mechanical parts and electronic assemblies to our subcontractors and dedicating substantial resources to the manufacture of new epitaxial equipment prior to any commitment to purchase by a customer and without generating any sales from our customers. In addition, orders already placed by a customer are subject to cancellation. In most cases, revenue from the sale of our systems is not recognized until final customer acceptance. Customer acceptance may occur upon installation or in some cases, upon demonstrated system performance, which may take up to 120 days or more from the date of shipment. Our costs would increase and profitability would suffer if our customers delayed or canceled orders, or if we were unable to recognize revenue in a timely manner.
| We depend on system sales to a relatively small number of customers for a significant portion of our revenue, and if any of these customers were to stop or reduce their purchasing from us, it would adversely affect our sales and profitability. |
A loss or a significant reduction or delay in sales, particularly system sales, to any of our major customers could materially and adversely affect our profitability. During 1999, our top ten customers accounted for an aggregate of 46% of our sales. We expect that sales from a relatively small number of customers will account for an increasing portion of our sales, particularly with the introduction of our high volume production MBE systems which have relatively high average selling prices. Moreover, the market for commercial production MBE systems, such as our GEN2000 system, is currently estimated to be less than 50 potential customers. We do not have long-term or other non-cancelable commitments from our customers to purchase our systems and related components.
Our rapid growth may place a strain on our resources.
We are experiencing rapid growth and plan to expand our manufacturing facilities, expand our product offering with MOCVD equipment and other compound semiconductor fabrication line equipment and process solutions, establish and develop a Process Integration Center, and consider further internal development as well as strategic joint ventures and acquisitions in connection with the development of new products and technologies. We believe that this growth could place a significant strain on our management, information technology and operating systems, financial and sales staff and other current employees, who may be forced to spend significant amounts of their time implementing these strategic initiatives. In addition, our manufacturing capacity and employee base may not be adequate to support increases in orders for our products, and we may not be able to expand quickly
9
| We rely upon a limited number of suppliers for the purchase of certain raw materials required for the manufacture of our products, and our production could suffer if they were to interrupt or discontinue supply. |
We purchase certain raw materials used to manufacture our epitaxial equipment and related components from a limited number of suppliers. For example, we purchase a material called tantalum from the only two primary sources in the world. Tantalum is currently in short supply, which has resulted in price increases and may cause future shortages. In addition, there are only three primary sources of pyrolytic boron nitride in the world, and we currently use two of them. Our manufacturing operations depend upon obtaining adequate supplies of these and other raw materials on a timely basis, and our operations and ability to satisfy our customer obligations could suffer if tantalum or any other raw material we use became unavailable due to a shortage, commercially unreasonable pricing, disruption or termination of our relationship with the supplier or any other reason. We expect the demand for these raw materials to grow in the future, which may result in additional shortages or price increases. We do not have long-term supply agreements with our raw material suppliers and purchase such materials on a purchase order basis.
| Our business depends on our intellectual property rights, and if we are unable to adequately protect them, our competitive position will suffer. |
Our success depends in part on our proprietary technology. We attempt to protect our intellectual property rights primarily through patents, trade secrets and non-disclosure agreements. However, we might not be able to protect some of our technology, and competitors might be able to develop similar technology independently. Our patents might not be sufficiently broad to protect our technology and any existing or future patents might be challenged, invalidated or circumvented. Additionally, our rights under our patents may not provide competitive advantages. If parties to non-disclosure agreements with us breach these agreements, we may not have an adequate remedy. The laws of certain foreign countries might not afford our intellectual property the same protection as do the laws of the United States and as a result, we may have an increasingly difficult time adequately protecting our intellectual property rights as our sales in foreign countries grow. If we are unable to pay royalties under certain license agreements for patents we use in the manufacture of effusion cells, our rights to use those patents and manufacture these products could be harmed. We may also need to engage in litigation in the future to enforce or protect our intellectual property rights or to defend against claims of invalidity, and we may incur substantial costs as a result.
| If we become subject to intellectual property infringement claims by third parties, we could incur unanticipated expenses and be prevented from providing our products and services. |
Some of our current or future products could infringe patents or proprietary rights of others. Litigation may be necessary to defend ourselves against claimed infringement of the rights of others or to determine the scope or validity of the proprietary rights of others. Litigation, whether or not meritorious, could be expensive and divert management resources from operating our company. Furthermore, a party making a claim against us could secure a judgment awarding substantial damages, as well as injunctive or other equitable relief that could block our ability to provide products or services, unless we obtain a license to such technology. Licenses for any intellectual property of third parties that might be required for our products or services might not be available on commercially reasonable terms.
10
| If we fail to identify and complete future acquisitions, our ability to expand, increase our revenues and execute our long-term strategy would be harmed. |
One of our strategies is to expand by acquiring other businesses and technologies. However, we currently have no commitments or agreements with respect to any acquisition. We might not be able to successfully identify, negotiate or finance any acquisition, which could diminish our ability to expand our business and remain competitive. We cannot predict whether or when any prospective acquisition candidate will become available or the likelihood that any acquisition will be completed.
| Future acquisitions could be difficult to integrate, disrupt our business and dilute shareholder value. |
In order to expand our product and service offerings and execute our growth strategy, we may acquire products, technologies or businesses that we believe are complementary. Acquisitions involve numerous risks, including:
| • | diversion of management’s attention away from our core, day-to-day business since these transactions may be time-consuming and place strain on our resources; | |
| • | inefficient integration of the acquired business or technology, including difficulties in the integration of the operations, information systems, services, products and personnel, thereby reducing the anticipated benefits of the acquisition; | |
| • | potentially dilutive issuances of equity securities; | |
| • | the potential loss of key employees of the acquired company; | |
| • | our inability to maintain the goodwill of the acquired businesses; | |
| • | the incurrence of additional debt; | |
| • | the assumption of known and unknown liabilities; and | |
| • | the write-off of development costs, and the amortization of expenses related to goodwill and other intangible assets and charges against earnings. |
Any of the above risks, if they occur, could harm our business.
| Because a large percentage of our sales are from foreign customers, export and foreign currency risks may disproportionately affect our revenues. |
Sales to our customers located outside the United States accounted for approximately 32% of our sales in 1997, 35% in 1998, 40% in 1999, and 38% for the six months ended June 30, 2000. Sales to our customers in the Asia-Pacific region and Europe represent the majority of our international sales. We believe that international sales will continue to account for a significant percentage of our sales and will continue to expose us to the following risks that may reduce our sales or increase our costs, thereby negatively affecting our profitability:
| • | political and economic instability may inhibit export of our products and services; | |
| • | shipping and installation costs of our systems may increase; | |
| • | we may experience difficulties in the timeliness in collection of foreign accounts receivables and may be forced to write off such receivables from a foreign customer; |
11
| • | a strong dollar may make the price of our epitaxial products and services less attractive to a foreign purchaser who may decide to postpone making the capital expenditure or purchase the required products or services from a foreign competitor; | |
| • | tariffs and other barriers may make our products and services less price competitive; | |
| • | we may have difficulty staffing and managing our international operations; | |
| • | we may have difficulty or be delayed in obtaining the export licenses required to export some of our products to various destinations, such as China and Taiwan; | |
| • | the laws of certain foreign countries may not adequately protect our patents and other intellectual property; and | |
| • | our results of operations can be hurt by fluctuations in foreign currency exchange rates. |
| Any disruption in the operation of our single manufacturing facility or delays in expanding our manufacturing capacity could harm our sales and profitability. |
We assemble and test all of our products in our manufacturing facility in St. Paul, Minnesota. The assembly and testing of our products is a highly complex and precise process, requiring sophisticated and costly equipment and a specially designed facility. As a result, a disruption in the operation of our manufacturing facility, whether due to technical or labor difficulties, utility failures, destruction or damage to the facility as a result of tornado, fire or any other reason, could harm our sales and profitability. In addition, we are in the process of expanding our manufacturing facility and delays in the construction of that facility, adjacent to our current manufacturing facility, could harm our future growth.
| Unsuccessful control of hazardous materials could result in costly remediation fees, penalties and damages under environmental, health and safety regulations. |
We are subject to various federal, state and local environmental, health and safety laws and regulations. We maintain small amounts of acids and other chemical agents for our manufacturing and service operations. In addition, we provide chemical decontamination and cleaning services on our customers’ MBE systems and related components that involve the handling, and in some cases disposal, of arsenic or phosphorus-based compounds and other hazardous materials. Operation and development of epitaxial equipment could result in the release of hazardous materials. In addition, MOCVD systems use larger amounts of hazardous materials than MBE systems. If our control systems and safety procedures are unsuccessful in preventing release of these materials into the environment or exposure of our personnel to occupational hazards, we may be subject to liability under environmental, health and safety laws and could incur significant expenses.
| We may be exposed to product liability for our products which could harm our sales and profitability. |
Our products are used by our customers under extreme temperature, vacuum and pressure conditions to manufacture compound semiconductors. If our products fail or are defective, we could face business interruption, personal injury or product liability claims. In addition, we outsource the fabrication of certain parts and sub-assemblies of our equipment and components, which could expose us to additional product liability for defective or sub-standard parts and sub-assemblies manufactured by our suppliers. If an exceptional occurrence of damage or personal injury were to occur, our
12
Risks Related to this Offering
| Existing shareholders may use their controlling interest in Applied Epi to take actions not supported by minority shareholders. |
After this offering, it is anticipated that our officers and directors will beneficially own or control, directly or indirectly, % of our shares of common stock. As a result, if some of these existing shareholders choose to act together, they will have the ability to control all matters submitted to our shareholders for approval, including the election and removal of directors and the approval of any business combinations. This may delay or prevent an acquisition or cause the market price of our stock to decline. Our directors and officers may have interests different from yours. For example, they may be more interested than other investors in selling our company or pursuing different business strategies.
| Our stock price may be volatile and you may not be able to resell your shares at or above the initial public offering price. |
There has been no public market for our common stock prior to this offering. The initial public offering price for our common stock will be determined through negotiations between the underwriters and us. This initial public offering price will vary from the market price of our common stock after the offering. If you purchase shares of common stock, you may not be able to resell those shares at or above the initial public offering price. The market price of our common stock may fluctuate significantly in response to many factors, some of which are beyond our control, including the following:
| • | actual or anticipated fluctuations in our annual and quarterly operating results; | |
| • | changes in market valuations of companies in the compound semiconductor industry and in the industries which the compound semiconductor industry serves; | |
| • | changes in financial estimates by securities analysts; | |
| • | variations in our operating results which may cause us to fail to meet analysts’ or investors’ expectations; | |
| • | announcements by us or our competitors of significant technological innovations, contracts, acquisitions, strategic partnerships, joint ventures or capital commitments; | |
| • | additions or departures of key personnel; | |
| • | future sale of equity or debt securities; and | |
| • | general economic, industry and market conditions. |
In addition, the stock market has experienced extreme volatility that often has been unrelated to the performance of particular companies. These market fluctuations may cause our stock price to fall regardless of our performance. In the past, some companies that have experienced volatility in the market price of their stock have faced securities class action litigation. If we were faced with securities class action litigation, it could result in substantial costs and a diversion of management’s attention and resources.
13
| We may issue new equity securities to meet our future capital requirements and shareholders may experience additional dilution. |
We may be required, or could elect, to seek funding if we acquire businesses, products or technologies, or fund internal development efforts. In the event we are required to raise funds we may not be able to do so on favorable terms, if at all. Further, if we issue new equity securities, shareholders may experience dilution or the holders of new equity securities may have rights, preferences or privileges senior to those of existing holders of our common stock.
| Substantial future sales of our common stock by existing shareholders in the public market may depress our stock price. |
Our current shareholders hold a substantial number of shares, which they will be able to sell in the public market in the near future. Sales of a substantial number of shares of our common stock after this offering could cause our stock price to fall, impairing our ability to raise capital through the sale of additional stock.
| You will experience immediate and substantial dilution in the book value of your shares. |
The initial public offering price is substantially higher than the book value per share of our outstanding common stock immediately after the offering. Accordingly, if you purchase common stock in the offering, you will incur immediate dilution of approximately $ in the book value per share of our common stock from the price you pay for our common stock.
| The provisions of our charter documents may inhibit bids to acquire us that a shareholder may believe are desirable, and the market price of our common stock may be lower as a result. |
Our board of directors has the authority to issue up to 25,000,000 shares of preferred stock. Our board of directors can fix the price, rights, preferences, privileges and restrictions of the preferred stock without any further vote or action by our shareholders. The issuance of shares of preferred stock may delay or prevent a change in control transaction. As a result, the market price of our common stock and the voting and other rights of our shareholders may be adversely affected. The issuance of preferred stock may result in the loss of voting control to other shareholders.
Our charter documents also contain other provisions which may discourage, delay or prevent a merger or acquisition of us, including:
| • | a classified board of directors, with each class of directors subject to reelection every three years, which limits our shareholders’ ability to quickly change a majority of the board of directors; | |
| • | a 70% shareholder vote requirement for the removal of a director by shareholders; and | |
| • | procedural and other requirements that could make it difficult for shareholders to effect certain corporate actions. |
We are also subject to Sections 302A.671 (Control Share Acquisition Act) and 302A.673 (Business Combination Act) of the Minnesota Business Corporation Act which may have the effect of limiting third parties from acquiring significant amounts of our common stock without our approval.
These provisions could also have the effect of discouraging others from making tender offers for our common stock. As a result, these provisions may prevent the market price of our common stock
14
| We have broad discretion in how we use the proceeds of this offering, and we may not use these proceeds effectively. |
Our management has broad discretion in the use of the net proceeds of this offering and could spend the net proceeds in ways that may not yield a favorable return. We may also use the proceeds to acquire complementary businesses or technologies, although no specific acquisitions are currently planned. Until we need to use the proceeds of this offering, we plan to invest the net proceeds in short-term, interest-bearing securities.
This prospectus contains forward-looking statements that involve risks and uncertainties. These statements relate to future events or our future financial performance and include statements about our plans, objectives and services as well as our expectations and intentions. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential” or “continues” or the negative of such terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks outlined under “Risk Factors,” that may cause our or our industry’s actual results, levels of activity, performance or achievements expressed or implied by any forward-looking statements to differ materially from those predicted.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. We are under no duty to update any of the forward-looking statements after the date of this prospectus to conform these statements to actual results.
15
We estimate that the net proceeds from our sale of the shares of common stock in this offering will be approximately $ million, at an assumed initial public offering price of $ per share, after deducting estimated underwriting discounts and commissions and offering expenses payable by us. If the underwriters’ over-allotment option is exercised in full, we estimate that our net proceeds will be approximately $ million.
We are conducting this offering primarily to create a public market for our common stock, facilitate future access by us to public equity markets and increase our visibility in the marketplace. We presently intend to use a portion of the net proceeds of this offering for the following purposes:
| • | approximately $5.0 million for construction and development costs of our Process Integration Center; | |
| • | approximately $5.0 million to expand our manufacturing facilities; | |
| • | approximately $6.0 million for distributions to our current shareholders in connection with the termination of our S corporation status; and | |
| • | approximately $ million for distributions to holders of performance units in connection with the termination of our Employee Performance Stock Ownership Plan upon completion of this offering. |
We may use a portion of the net proceeds to acquire or make investments in complementary products, technologies or businesses. We have no present commitments or agreements with respect to any of these kinds of acquisitions or investments, although they are an integral part of our growth strategy.
We have no specific plans at this time for the use of the remaining proceeds and expect to use these proceeds for working capital and general corporate purposes. Our management will have broad discretion concerning the allocation and use of a significant portion of the net proceeds of this offering. Pending such uses, we plan to invest the net proceeds in short-term, interest-bearing securities.
16
We intend to retain any future earnings for investment in the development and expansion of our business other than the dividends we intend to pay as discussed below under “S Corporation Status.” In addition, terms of our credit agreement with U.S. Bank National Association prohibit us from paying dividends or making any cash distributions on our capital stock, other than distributions to allow our shareholders to pay their S corporation taxes, without the bank’s prior written consent.
Beginning in 1997, we have been treated for federal and state income tax purposes as an S corporation under Subchapter S of the Internal Revenue Code and comparable state laws. As a result, our earnings have been taxed for federal and state income tax purposes directly to our shareholders rather than to us. In connection with this offering, we will convert from an S corporation to be taxed as a C corporation. As a result of the termination of our S corporation status, we will record a net deferred tax asset and corresponding income tax benefit effective upon the termination date. The amount of the deferred tax asset would have been approximately $978,000 if the termination date had been June 30, 2000. The actual amount will be determined after giving effect to our operating results through the termination date.
We expect to distribute approximately $4.0 million from the net proceeds of this offering to our S corporation shareholders, representing our S corporation income that had been taxed to our shareholders and not previously distributed to them as of December 31, 1999, plus an additional amount equal to our estimated taxable income earned during 2000 prior to the conversion to a C corporation. We estimate that this additional amount would have been approximately $2.0 million if the conversion to a C corporation had occurred on June 30, 2000. The actual amount will be determined after giving effect to our operating results through the S corporation termination date.
17
The following table sets forth our actual, pro forma and pro forma as adjusted capitalization as of June 30, 2000. Our pro forma as adjusted capitalization gives effect to the sale of shares of common stock in this offering at an assumed initial public offering price of $ per share, after deducting estimated underwriting discounts and commissions and offering expenses and the payment of the previously undistributed S corporation earnings. You should read this table in conjunction with our consolidated financial statements and the accompanying notes included elsewhere in this prospectus, “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| June 30, 2000 | ||||||||||||||
| Pro Forma | ||||||||||||||
| Actual | Pro Forma(1) | As Adjusted(1) | ||||||||||||
| (in thousands, except share data) | ||||||||||||||
| Cash and cash equivalents | $ | 1,885 | $ | 1,885 | $ | |||||||||
| Long-term debt, less current portion | 5,107 | 5,107 | ||||||||||||
| Shareholders’ equity: | ||||||||||||||
| Preferred stock, $0.01 par value, 25,000,000 shares authorized, no shares issued and outstanding, actual, pro forma and pro forma as adjusted | — | — | — | |||||||||||
| Common stock, $0.01 par value, 100,000,000 shares authorized, 21,602,980 shares issued and outstanding, actual; 100,000,000 shares authorized, shares issued and outstanding, as adjusted | 216 | 216 | ||||||||||||
| Additional paid-in capital(2) | 18,073 | 18,984 | ||||||||||||
| Deferred stock-based compensation | (10,201 | ) | (10,201 | ) | ||||||||||
| Retained earnings(2) | 911 | 978 | ||||||||||||
| Total shareholders’ equity | 8,999 | 9,977 | ||||||||||||
| Total capitalization | $ | 14,106 | $ | 15,084 | $ | |||||||||
Outstanding shares in the table above:
| • | exclude, as of September 25, 2000, an aggregate of 6,000,000 shares of our common stock currently reserved for issuance under our 1993 Stock Option Plan, of which 1,952,248 shares are subject to outstanding options at a weighted average exercise price of $4.92 per share; | |
| • | exclude, as of September 25, 2000, an aggregate of 6,000,000 shares of our common stock currently reserved for issuance under our 2000 Stock Plan, of which 242,000 shares are subject to outstanding options at an exercise price of $12.50 per share; and | |
| • | exclude conversion of phantom stock units issued under our Employee Performance Stock Ownership Plan into 621,482 shares of our common stock and exclude an aggregate of 734,388 units available for future issuance under the Plan. |
| (1) | Reflects adjustment to account for the deferred tax asset described in “S Corporation Status.” See Note 3 of Notes to Consolidated Financial Statements. |
| (2) | Retained earnings have been recapitalized to additional paid-in capital upon our conversion to a C corporation. The amount capitalized is net of the adjustment necessary to record the estimated deferred tax asset. |
18
The net tangible book value of our common stock as of June 30, 2000 was approximately $8.3 million, or approximately $0.39 per share. Net tangible book value per share represents the amount of our shareholders’ equity, less goodwill and other intangible assets, divided by the total number of shares of common stock outstanding. Dilution per share to new investors represents the difference between the amount per share paid by purchasers of shares of common stock in this offering and the net tangible book value per share of our common stock immediately after the completion of this offering.
After giving effect to the sale of the shares of common stock offered by us at an assumed initial public offering price of $ per share and after deducting estimated underwriting discounts and commissions and offering expenses, our net tangible book value as of June 30, 2000 would have been approximately $ million or approximately $ per share. This represents an immediate increase in net tangible book value of $ per share to existing shareholders and an immediate dilution in net tangible book value of approximately $ per share to new investors, or approximately % of the assumed initial public offering price of $ per share. The following table illustrates this per share dilution:
| Assumed initial public offering price per share | $ | ||||||||
| Net tangible book value per share as of June 30, 2000 | $ | 0.39 | |||||||
| Increase in net tangible book value per share attributable to new investors | |||||||||
| Net tangible book value per share after this offering | |||||||||
| Dilution per share to new investors | $ | ||||||||
The following table illustrates as of June 30, 2000 the difference between the number of shares of common stock purchased from us, the total consideration paid to us and the average price paid by existing shareholders and by the new investors purchasing our common stock in this offering, before deducting the estimated underwriting discounts and commissions and offering expenses:
| Shares Purchased | Total Consideration | ||||||||||||||||||||
| Average Price | |||||||||||||||||||||
| Number | Percent | Amount | Percent | Per Share | |||||||||||||||||
| Existing shareholders | |||||||||||||||||||||
| New investors | $ | $ | |||||||||||||||||||
| Total | 100.0 | % | $ | 100.0 | % | $ | |||||||||||||||
The foregoing discussion and table are based on actual shares outstanding on June 30, 2000 and assume no exercise of any stock options outstanding as of June 30, 2000. As of June 30, 2000, there were options outstanding to purchase 1,586,248 shares of common stock at a weighted average exercise price of $3.55 share. To the extent any of these options are exercised, there will be further dilution to investors. See “Capitalization,” “Management — Stock Plans,” “Description of Capital Stock” and Note 8 of Notes to Consolidated Financial Statements included elsewhere in this prospectus.
19
The selected statement of operations data presented below for the years ended December 31, 1997, 1998 and 1999, and the balance sheet data as of December 31, 1998 and 1999 are derived from our consolidated financial statements (presented on an “as if pooling” basis; see Note 1 of Notes to Consolidated Financial Statements), which have been audited and are included at the end of this prospectus. The selected statement of operations data for the years ended December 31, 1995 and 1996, and the balance sheet data as of December 31, 1995, 1996 and 1997, are derived from our consolidated financial statements, which have been audited and are not included in this prospectus. The selected statement of operations data for the six months ended June 30, 1999 and 2000, and the balance sheet data as of June 30, 2000, are derived from our consolidated financial statements, which have not been audited and are included at the end of this prospectus. In the opinion of management, the unaudited consolidated financial statements have been prepared on the same basis as the audited consolidated financial statements and include all adjustments (consisting only of normal recurring adjustments) necessary for a fair presentation of our results of operations for such periods and financial condition as of that date. The pro forma as adjusted balance sheet data reflect the sale by us of shares of common stock offered hereby at an assumed initial public offering price of $ per share after deducting the estimated underwriting discounts and commissions and offering expenses payable by us and the payment of the previously undistributed S corporation earnings.
The historical results presented below are not necessarily indicative of the results to be expected for any future period. The selected financial data set forth below is qualified in its entirety by, and should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and notes thereto included at the end of this prospectus.
| Six Months Ended | ||||||||||||||||||||||||||||||
| Year Ended December 31, | June 30, | |||||||||||||||||||||||||||||
| 1995 | 1996 | 1997 | 1998 | 1999 | 1999 | 2000 | ||||||||||||||||||||||||
| (in thousands, except per share data) | ||||||||||||||||||||||||||||||
| Statement of Operations Data: | ||||||||||||||||||||||||||||||
| Sales | $ | 12,641 | $ | 12,088 | $ | 11,854 | $ | 15,533 | $ | 15,389 | $ | 6,849 | $ | 10,180 | ||||||||||||||||
| Cost of sales(1) | 6,561 | 6,710 | 6,180 | 8,686 | 8,579 | 3,885 | 5,237 | |||||||||||||||||||||||
| Gross profit | 6,080 | 5,378 | 5,674 | 6,847 | 6,810 | 2,964 | 4,943 | |||||||||||||||||||||||
| Operating expenses: | ||||||||||||||||||||||||||||||
| Selling(2) | 978 | 918 | 1,498 | 1,617 | 1,829 | 760 | 1,054 | |||||||||||||||||||||||
| General and administrative(3) | 2,012 | 1,105 | 1,146 | 1,108 | 1,043 | 532 | 1,086 | |||||||||||||||||||||||
| Research and development(4) | 816 | 1,671 | 1,458 | 717 | 472 | 235 | 747 | |||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | — | — | 7,224 | |||||||||||||||||||||||
| Total operating expenses | 3,806 | 3,694 | 4,102 | 3,442 | 3,343 | 1,527 | 10,111 | |||||||||||||||||||||||
| Income (loss) from operations | 2,274 | 1,684 | 1,572 | 3,405 | 3,466 | 1,437 | (5,168 | ) | ||||||||||||||||||||||
| Other income (expense), net | 213 | 69 | (42 | ) | 1,666 | 1,626 | 1,627 | (93 | ) | |||||||||||||||||||||
| Income (loss) before income taxes | 2,487 | 1,753 | 1,530 | 5,071 | 5,092 | 3,064 | (5,261 | ) | ||||||||||||||||||||||
| Provision for income taxes | 1,081 | 302 | 213 | — | — | — | — | |||||||||||||||||||||||
| Net income (loss) | $ | 1,406 | $ | 1,451 | $ | 1,317 | $ | 5,071 | $ | 5,092 | $ | 3,064 | $ | (5,261 | ) | |||||||||||||||
| Historical net income (loss) | 5,092 | (5,261 | ) | |||||||||||||||||||||||||||
| Pro forma provision for income taxes(5) | 1,616 | 517 | ||||||||||||||||||||||||||||
| Pro forma net income (loss)(5) | 3,476 | (5,778 | ) | |||||||||||||||||||||||||||
| Pro forma net income (loss) per common share (basic and diluted)(5) | $ | 0.16 | $ | (0.26 | ) | |||||||||||||||||||||||||
| Pro forma weighted average common shares (basic and diluted)(5) | 22,065 | 22,065 | ||||||||||||||||||||||||||||
20
| June 30, 2000 | ||||||||||||||||||||||||||||||||
| December 31, | ||||||||||||||||||||||||||||||||
| Pro | Pro Forma As | |||||||||||||||||||||||||||||||
| 1995 | 1996 | 1997 | 1998 | 1999 | Actual | Forma(6) | Adjusted | |||||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||||||||||
| Balance Sheet Data: | ||||||||||||||||||||||||||||||||
| Cash and cash equivalents | $ | 3,405 | $ | 1,046 | $ | 1,678 | $ | 1,799 | $ | 1,478 | $ | 1,885 | $ | 1,885 | ||||||||||||||||||
| Working capital | 3,767 | 2,211 | 978 | (4,810 | ) | 4,715 | 4,063 | 4,502 | ||||||||||||||||||||||||
| Total assets | 11,152 | 6,653 | 7,185 | 8,371 | 16,290 | 21,832 | 22,810 | |||||||||||||||||||||||||
| Long-term debt, less current portion | — | — | — | — | 5,018 | 5,107 | 5,107 | |||||||||||||||||||||||||
| Total shareholders’ equity (deficit) | 4,913 | 3,254 | 1,910 | (4,107 | ) | 7,254 | 8,999 | 9,977 | ||||||||||||||||||||||||
| (1) | Excludes $84,029 for the six months ended June 30, 2000 reported below as amortization of deferred stock-based compensation. |
| (2) | Excludes $205,307 for the six months ended June 30, 2000 reported below as amortization of deferred stock-based compensation. |
| (3) | Excludes $6,824,340 for the six months ended June 30, 2000 reported below as stock-based compensation. |
| (4) | Excludes $110,437 for the six months ended June 30, 2000 reported below as amortization of deferred stock-based compensation. |
| (5) | Applied Epi operated as an S corporation since 1997 and will terminate such status in connection with this offering. See Note 3 of Notes to Consolidated Financial Statements for information concerning the computation of pro forma net income (loss) and pro forma net income (loss) per share. |
| (6) | Reflects adjustments to account for the deferred tax asset and S corporation distributions payable described in “S Corporation Status.” See Note 3 of Notes to Consolidated Financial Statements. |
21
The following discussion and analysis should be read in conjunction with “Selected Financial Data” and our consolidated financial statements and the related notes included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in the forward-looking statements as a result of certain factors including the risks discussed in “Risk Factors” and elsewhere in this prospectus.
Overview
We are a leading provider of epitaxial equipment and related components used to produce compound semiconductors for the fiber optic and wireless markets, as well as for other consumer applications. For 14 years, we have offered products and services designed to cost-effectively meet the increasingly demanding production requirements of the global high-performance communications infrastructure. We sell our products to compound semiconductor manufacturers, epiwafer suppliers and research and academic institutions. Our product line consists of both high and low volume production MBE systems, research MBE systems and key components of these systems. We are also currently developing a production MOCVD system.
We began manufacturing MBE equipment and related components in 1986 with our introduction of material specific effusion cells. Since then, we have continued to expand our product line to meet the increasing demands of the compound semiconductor industry. We began development of our first low volume production MBE system in 1987 and sold the first system in 1989. In 1988, we invented our solid source MBE valved cracker products. In December 1993, we enhanced our capabilities in MBE technology by acquiring the Modular GENII technology from IMBE, Inc., a subsidiary of Intevac, Inc. In 1995, we invented our SUMO effusion cells and also acquired Superior Vacuum Technology, Inc. (formerly a division of Perkin Elmer). This acquisition allowed us to expand our MBE technology capabilities with vacuum and robot design technologies, which have in part enabled us to develop our automated UHV multiwafer handling cluster tool.
In 1997, we began developing a high volume production MBE system and also further expanded our effusion cell product line by inventing our corrosive series valved cracker products. From 1998 and through June 1999, we loaned funds to Spectracom, Inc. (a related party) for the development of high power 980nm pump laser technology. Our involvement with Spectracom enhanced our understanding of fiber optic technologies. Spectracom was sold to ADC Telecommunications, Inc. in June 1999. Our first high volume production MBE system, the GEN2000, was introduced in January 2000 and the first system was shipped in the third quarter of 2000. We began developing our first production MOCVD system in 2000 and expect to introduce it in the second half of 2001.
Historically, we have derived our revenue from the sale of our components, such as effusion cells and crucibles, and low volume production and research MBE systems. However, while these products are expected to continue to generate significant revenues for us, the majority of our current backlog consists of orders for high volume production MBE systems, including the GEN2000 and GEN200.
Our United States sales efforts are managed from our corporate headquarters in St. Paul, MN, and sales offices in Boston, MA and Santa Clara, CA. Our European sales efforts are managed from our office in Greater Manchester, United Kingdom. Our Asia-Pacific sales efforts are managed by our Asia-Pacific sales manager based at our St. Paul, MN, headquarters. We also have sales representatives located in Taiwan, Singapore, China, Korea, India, France and Japan. A significant portion of our sales is to customers outside the United States. International sales accounted for approximately 32% of our sales in 1997, 35% in 1998, 40% in 1999 and 38% for the six months ended June 30, 2000.
22
In December 1999, the Securities and Exchange Commission issued Staff Accounting Bulletin (SAB) No. 101 — Revenue Recognition in Financial Statements. SAB 101 addresses several issues, including the timing for recognizing revenue from sales that involve obligations on the part of the vendor subsequent to shipment or transfer of title. In accordance with SAB 101, we recognize revenue when our obligation to our customers is satisfied. For high volume and certain low volume production and research systems, our obligation is satisfied based upon final customer acceptance. Customer acceptance is determined at either installation or, in some cases, demonstrated system performance some time after installation. The post shipment obligation associated with the sale of our high and low volume production and research systems is expected to range from 30 to 120 days. For our performance products and the remaining systems, we recognize revenues when the products are shipped. There are no customer acceptance criteria associated with the sale of performance products.
Typically, we require that buyers of our high and low volume production and research systems pay approximately 20% of the price of the system upon submission of their order, 50% upon shipment and 30% upon final customer acceptance. In limited cases, final payment is due after demonstrated system performance.
Our operating expenses are classified into four general categories: selling; general and administrative; research and development; and amortization of deferred stock-based compensation. Selling expenses consist primarily of salaries and other related costs for sales and marketing personnel, certain direct sales commission and other related activities. General and administrative expenses consist primarily of salaries, professional services and other administrative expenses. Research and development expenses consist primarily of salaries, benefits and materials. Amortization of deferred stock-based compensation represents the amortization over the related period of the difference between the exercise price of options granted and the estimated fair market value of the underlying common stock on the date of grant. We have amortized deferred compensation charges of $1.7 million for the six months ended June 30, 2000. In August 2000, vesting was accelerated on 899,448 options originally issued in April 2000 to two of our officers. This accelerated vesting will result in a new measurement date and a non-cash compensation charge in the quarter ended September 30, 2000 of approximately $7.4 million. As a result of the above, unamortized deferred stock-based compensation charges will reduce future earnings or increase future losses by the following annual amounts: $10.1 million in 2000; $1.2 million in 2001; $0.5 million in 2002; and $0.1 million in 2003.
In April 2000, our majority shareholder sold 569,112 shares of our common stock to four current employees at $1.40 per share. Accordingly, a charge to compensation expense was made to the extent the stock price was below the deemed fair value of the shares sold and a corresponding entry was made to record additional paid-in capital of $5.5 million in the six-month period ended June 30, 2000.
In connection with the conversion of phantom stock units issued under our Employee Performance Stock Ownership Plan into cash and 621,482 shares of our common stock, we expect to record significant compensation expense relating to stock-based compensation. This amount will be based on the initial public offering price and will be recorded in the quarter in which we complete our initial public offering.
We have two subsidiaries, Applied Epi Europe, Inc. and Applied Epi International, Inc. Applied Epi International was incorporated in 1992 as an Interest Charge Domestic International Sales Corporation (IC-DISC) to perform foreign sales activities and to obtain tax benefits related to our foreign sales. Applied Epi Europe was incorporated in 1993 for the purpose of sales and distribution of our products in Europe.
23
Results of Operations
The following table sets forth the relationship between various components of operations, stated as a percentage of sales, for each of the periods indicated. Our historical financial data for 1997, 1998 and 1999 were derived from, and should be read in conjunction with, our audited consolidated financial statements and the related notes included at the end of this prospectus. The historical financial data for the six months ended June 30, 1999 and June 30, 2000 were derived from our unaudited consolidated financial statements, which, in the opinion of management, reflect all adjustments necessary for the fair presentation of the financial condition and results of operations for such periods.
Results of Operations
| Six Months | |||||||||||||||||||||||
| Ended | |||||||||||||||||||||||
| Year Ended December 31, | June 30, | ||||||||||||||||||||||
| 1997 | 1998 | 1999 | 1999 | 2000 | |||||||||||||||||||
| Percentage of Sales: | |||||||||||||||||||||||
| Sales | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | |||||||||||||
| Cost of sales(1) | 52.1 | 55.9 | 55.7 | 56.7 | 51.4 | ||||||||||||||||||
| Gross profit | 47.9 | 44.1 | 44.3 | 43.3 | 48.6 | ||||||||||||||||||
| Operating expenses: | |||||||||||||||||||||||
| Selling(1) | 12.6 | 10.4 | 11.9 | 11.1 | 10.4 | ||||||||||||||||||
| General and administrative(1) | 9.7 | 7.1 | 6.8 | 7.8 | 10.7 | ||||||||||||||||||
| Research and development(1) | 12.3 | 4.6 | 3.1 | 3.4 | 7.3 | ||||||||||||||||||
| Stock-based compensation | — | — | — | — | 71.0 | ||||||||||||||||||
| Total operating expenses | 34.6 | 22.2 | 21.7 | 22.3 | 99.3 | ||||||||||||||||||
| Income (loss) from operations | 13.3 | 21.9 | 22.5 | 21.0 | (50.8 | ) | |||||||||||||||||
| Interest and other income (expense), net | (0.4 | ) | 10.7 | 10.6 | 23.8 | (0.9 | ) | ||||||||||||||||
| Income (loss) before income taxes | 12.9 | 32.6 | 33.1 | 44.7 | (51.7 | ) | |||||||||||||||||
| Provision for income taxes(2) | 1.8 | — | — | — | — | ||||||||||||||||||
| Net income (loss) | 11.1 | % | 32.6 | % | 33.1 | % | 44.7 | % | (51.7 | )% | |||||||||||||
| (1) | Excludes stock-based compensation. |
| (2) | Applied Epi operated as an S corporation since 1997 and will terminate such status in connection with this offering. |
Six Months Ended June 30, 2000 Compared to Six Months Ended June 30, 1999
Sales
Sales increased $3.4 million, or 50.0%, to $10.2 million in the six months ended June 30, 2000, compared to $6.8 million in the same period in 1999. This increase was attributable to an increase of $1.9 million in the sales of our low volume production MBE systems and a $1.5 million increase in the sales of our performance products.
Gross profit
Gross profit increased $1.9 million, or 63.3%, to $4.9 million in the six months ended June 30, 2000, compared to $3.0 million in the same period in 1999. Gross margin for the first six months of 2000 increased to 48.6% compared to 43.3% for the same period in 1999, primarily due to the
24
Selling, general and administrative expenses
Selling, general and administrative expenses increased $0.8 million, or 61.5%, to $2.1 million in the six months ended June 30, 2000, compared to $1.3 million in the same period in 1999. Additional salaries, benefits and office expenses associated with an increase in headcount accounted for $0.5 million of this increase. Higher professional fees primarily related to a resolved litigation matter, as well as increased costs in connection with marketing expenditures related to our GEN2000 system accounted for the remainder of the increase. Selling, general and administrative costs, as a percentage of sales, increased to 21.1% in the first six months of 2000 from 18.9% in the same period in 1999 primarily due to the increase of these items. We anticipate that selling, general and administrative costs in the remainder of 2000 and for 2001 will increase in absolute dollars as we hire additional administrative, sales and marketing personnel and expand our facilities.
Research and development expenses
Research and development expenses increased $0.5 million to $0.7 million in the six months ended June 30, 2000, compared to $0.2 million in the same period in 1999. Research and development costs, as a percentage of sales, increased to 7.3% in the first six months of 2000 from 3.4% in the same period in 1999. We expect that research and development as a percentage of sales will continue to increase as we continue to devote additional resources to our MBE and MOCVD product development.
Stock-based compensation
We have recorded deferred compensation for the difference between the exercise price of some stock option grants and the deemed fair market value of our common stock at the time of those grants. Deferred stock-based compensation is being amortized over the vesting periods of the applicable options, resulting in an expense of $1.7 million for the six months ended June 30, 2000.
In April 2000, our majority shareholder sold 569,112 shares of our common stock to four current employees at $1.40 per share. Accordingly, a charge to compensation expense was made to the extent the stock price was below the deemed fair value of the shares sold and a corresponding entry was made to record additional paid-in capital of $5.5 million in the six-month period ended June 30, 2000.
Interest and other income (expense), net
Net interest expense was $93,000 in the six months ended June 30, 2000, compared to net interest income of $1.6 million in the same period in 1999. The decrease reflects the reduction in interest income resulting from the June 1999 repayment to us of a loan by Spectracom, as well as increased interest expense in connection with the mortgage on our facilities.
Provision for income taxes
We did not record a provision for income taxes due to our S corporation status.
25
Net income (loss)
Net income decreased $8.4 million to a net loss of $5.3 million in the six months ended June 30, 2000 compared to net income of $3.1 million in the same period in 1999. This decrease is primarily attributable to a $7.2 million charge for deferred stock-based compensation. Pro forma net loss of $5.8 million in the six months ended June 30, 2000 includes a provision for income taxes of $0.5 million. This amount varies from a normal effective tax rate of approximately 40% due to the non-deductibility of the stock-based compensation charge and the tax benefit of IC-DISC sales.
Year Ended December 31, 1999 Compared to Year Ended December 31, 1998
Sales
Sales decreased $0.1 million, or 0.6%, to $15.4 million in 1999, compared to $15.5 million in 1998. Increased demand for our performance products was offset by the sale of a lower number of low volume production MBE systems.
Gross profit
Gross profit in 1999 remained flat at $6.8 million, compared to 1998. Gross margin for 1999 improved to 44.3% compared to 44.1% for 1998. The gross margin improvement in 1999 reflected an increase in revenues from sales of our higher margin performance products as a percentage of total revenues.
Selling, general and administrative expenses
Selling, general and administrative expenses increased $0.2 million, or 7.4%, to $2.9 million in 1999, compared to $2.7 million in 1998. This increase was due to increased advertising expenses and a modest foreign currency loss, partially offset by a decrease in office expenses. Selling, general and administrative costs, as a percentage of sales, increased to 18.7% in 1999 from 17.5% in 1998 primarily due to the increase of these items.
Research and development expenses
Research and development expenses decreased $0.2 million, or 28.6%, to $0.5 million in 1999, compared to $0.7 million in 1998. Research and development costs, as a percentage of sales, decreased to 3.1% in 1999 from 4.6% in 1998.
Interest and other income, net
Net interest and other income decreased $0.1 million to $1.6 million in 1999, compared to $1.7 million in net interest income in 1998. This decrease reflected the reduction in interest income resulting from the June 1999 repayment to us of a loan by Spectracom.
Provision for income taxes
We did not record a provision for income taxes due to our S corporation status.
26
Net income
Net income was approximately $5.1 million in both 1999 and 1998. Pro forma net income was approximately $3.8 million in 1999 and includes a provision for income taxes of approximately $1.3 million.
Year Ended December 31, 1998 Compared to Year Ended December 31, 1997
Sales
Sales increased $3.6 million, or 30.3%, to $15.5 million in 1998, compared to $11.9 million in 1997. This increase was primarily attributable to an increase of $2.1 million in the sales of our low volume production MBE systems and an increase of $1.5 million in the sales of our performance products.
Gross profit
Gross profit increased by $1.1 million, or 19.3%, to $6.8 million in 1998, compared to $5.7 million in 1997. Gross margin for 1998 declined to 44.1% compared to 47.9% for 1997, primarily as a result of the increase in low volume production MBE system sales as a percentage of total revenues, startup costs associated with the shipment of our first GENIII system, increased overhead associated with a move to new facilities in July 1998, and the write-off of materials purchased in connection with a canceled order.
Selling, general and administrative expenses
Selling, general and administrative expenses increased $0.1 million, or 3.8%, to $2.7 million in 1998, compared to $2.6 million in 1997. This increase was due to increased salaries, benefits, office expenses and facility costs, partially offset by a foreign currency gain. Selling, general and administrative costs, as a percentage of sales, decreased to 17.5% in 1998 from 22.3% in 1997 due to the increase in sales.
Research and development expenses
Research and development expenses decreased $0.8 million, or 53.3%, to $0.7 million in 1998, compared to $1.5 million in 1997. Research and development costs, as a percentage of sales, decreased to 4.6% in 1998 from 12.3% in 1997.
Interest and other income (expense), net
Net interest and other income was $1.7 million in 1998, compared to $42,000 in net interest and other expense in 1997. This change reflected interest income of $1.6 million associated with a loan by us to Spectracom.
Provision for income taxes
We did not record a provision for income taxes due to our S corporation status.
Net income
Net income increased $3.8 million, or 292.3%, to $5.1 million in 1998, compared to $1.3 million in 1997.
27
Quarterly Results of Operations
The following tables present consolidated statements of operations data in dollars and as a percentage of sales for the ten quarters ended June 30, 2000. In management’s opinion, this unaudited information has been prepared on the same basis as our audited consolidated financial statements appearing elsewhere in this prospectus. All adjustments which management considers necessary for the fair presentation of the unaudited information have been included in the quarters presented. Our results from operations may vary substantially from quarter to quarter. Accordingly, the operating results for any quarter are not necessarily indicative of results for any subsequent quarter, entire year or any future period.
| 1998 | 1999 | 2000 | |||||||||||||||||||||||||||||||||||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | ||||||||||||||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||||||||||||||||||||
| Sales | $ | 3,376 | $ | 3,911 | $ | 3,971 | $ | 4,274 | $ | 3,449 | $ | 3,400 | $ | 3,063 | $ | 5,476 | $ | 5,185 | $ | 4,995 | |||||||||||||||||||||
| Cost of sales(1) | 1,891 | 2,321 | 2,267 | 2,207 | 1,970 | 1,915 | 1,740 | 2,954 | 2,567 | 2,670 | |||||||||||||||||||||||||||||||
| Gross profit | 1,485 | 1,590 | 1,704 | 2,067 | 1,479 | 1,485 | 1,323 | 2,522 | 2,618 | 2,325 | |||||||||||||||||||||||||||||||
| Operating expenses(1) | 820 | 886 | 859 | 878 | 763 | 764 | 743 | 1,074 | 1,211 | 1,676 | |||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | — | — | — | — | — | 7,224 | |||||||||||||||||||||||||||||||
| Total operating expenses | 820 | 886 | 859 | 878 | 763 | 764 | 743 | 1,074 | 1,211 | 8,900 | |||||||||||||||||||||||||||||||
| Income (loss) from operations | 665 | 704 | 845 | 1,189 | 716 | 721 | 580 | 1,448 | 1,407 | (6,575 | ) | ||||||||||||||||||||||||||||||
| Interest and other income (expense), net | 23 | 562 | 180 | 901 | 144 | 1,483 | 8 | (9 | ) | (31 | ) | (62 | ) | ||||||||||||||||||||||||||||
| Income (loss) before income taxes | 688 | 1,266 | 1,025 | 2,090 | 860 | 2,204 | 588 | 1,439 | 1,376 | (6,637 | ) | ||||||||||||||||||||||||||||||
| Provision for income taxes | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
| Net income (loss) | $ | 688 | $ | 1,266 | $ | 1,025 | $ | 2,090 | $ | 860 | $ | 2,204 | $ | 588 | $ | 1,439 | $ | 1,376 | $ | (6,637 | ) | ||||||||||||||||||||
| (1) | Excludes stock-based compensation charge. |
| 1998 | 1999 | 2000 | |||||||||||||||||||||||||||||||||||||||
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | ||||||||||||||||||||||||||||||||
| (percentage of sales) | |||||||||||||||||||||||||||||||||||||||||
| Sales | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | |||||||||||||||||||||
| Cost of sales(1) | 56.0 | 59.3 | 57.1 | 51.6 | 57.1 | 56.3 | 56.8 | 53.9 | 49.5 | 53.5 | |||||||||||||||||||||||||||||||
| Gross profit | 44.0 | 40.7 | 42.9 | 48.4 | 42.9 | 43.7 | 43.2 | 46.1 | 50.5 | 46.5 | |||||||||||||||||||||||||||||||
| Operating expenses(1) | 24.3 | 22.7 | 21.6 | 20.5 | 22.1 | 22.5 | 24.3 | 19.6 | 23.4 | 33.6 | |||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | — | — | — | — | — | 144.6 | |||||||||||||||||||||||||||||||
| Total operating expenses | 24.3 | 22.7 | 21.6 | 20.5 | 22.1 | 22.5 | 24.3 | 19.6 | 23.4 | 178.2 | |||||||||||||||||||||||||||||||
| Income (loss) from operations | 19.7 | 18.0 | 21.3 | 27.8 | 20.8 | 21.2 | 18.9 | 26.4 | 27.1 | (131.6 | ) | ||||||||||||||||||||||||||||||
| Interest and other income (expense), net | 0.7 | 14.4 | 4.5 | 21.1 | 4.2 | 43.6 | 0.3 | (0.2 | ) | (0.6 | ) | (1.2 | ) | ||||||||||||||||||||||||||||
| Income (loss) before income taxes | 20.4 | 32.4 | 25.8 | 48.9 | 24.9 | 64.8 | 19.2 | 26.3 | 26.5 | (132.9 | ) | ||||||||||||||||||||||||||||||
| Provision for income taxes | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
| Net income (loss) | 20.4 | % | 32.4 | % | 25.8 | % | 48.9 | % | 24.9 | % | 64.8 | % | 19.2 | % | 26.3 | % | 26.5 | % | (132.9 | )% | |||||||||||||||||||||
| (1) | Excludes stock-based compensation charge. |
Most of our expenses, such as employee compensation, depreciation on our facilities and equipment are relatively fixed in the near term. In addition, our expense levels are based in part on our expectations regarding future sales. As a result, any shortfall in sales relative to our expectations
28
| • | the volume and timing of orders for our products, particularly our epitaxial production systems, which have relatively high average selling prices; | |
| • | the timing of customer acceptance of our epitaxial production systems. In most cases, we do not recognize revenue from our system contracts until the customer accepts the system. Customer acceptance may occur upon installation or in some cases, demonstrated system performance which is subsequent to installation; | |
| • | the timing of our announcement and introduction of new products and of similar announcements by our competitors; | |
| • | downturns in the market for our customers’ products; and | |
| • | regional economic conditions, particularly in Europe and the Asia-Pacific region, from which we derive significant sales. |
Liquidity and Capital Resources
We have historically financed our operations and capital requirements through cash flow from operating activities, long-term loans and borrowings under short-term lines of credit.
Operating activities
Cash flow provided by operations for the six month period ended June 30, 1999 was $2.0 million and for the six months ended June 30, 2000 was $1.9 million. The decrease was primarily due to higher levels of inventory and lower levels of net income, partly offset by higher deferred revenue in the first six months of 2000. We anticipate our levels of inventory to increase for the remainder of 2000 and in 2001. However, the amount of such increase is dependent upon the increase in our backlog.
Cash flow provided by operating activities totaled $1.3 million in 1997, $2.9 million in 1998 and $3.4 million in 1999. Net income, noncash charges and changes in working capital primarily accounted for the cash flow generated by operations. 1998 and 1999 operating cash flows also were impacted by the amortization of a discount on a note receivable from a related party.
Investing activities
Cash flow provided by investing activities was $7.8 million for the six months ended June 30, 1999 and cash flow used in investing activities was $2.9 million for the six months ended June 30, 2000. Acquisitions of property and equipment totaled $21,000 for the six months ended June 30, 1999 and $2.9 million for the six months ended June 30, 2000. Significant capital expenditures in 2000 included $2.3 million for the purchase of land adjacent to our current facilities, as well as additions of manufacturing equipment and facilities.
Cash flow used in investing activities totaled $2.4 million in 1997 and $5.6 million in 1998, while cash provided by investing activities was $0.5 million in 1999. Acquisitions of property and equipment totaled $0.1 million in 1997, $0.1 million in 1998 and $7.3 million in 1999. In December 1999, we purchased our facility and an adjacent lot for $6.9 million. Most of our capital expenditures are for facilities, manufacturing equipment and computer and communications equipment. Historically, advances to related parties were also included in cash flows from investing activities. We expect to
29
Financing activities
Financing activities in the six months ended June 30, 1999 used cash of $9.4 million and in the six months ended June 30, 2000 provided cash of $1.3 million.
Cash provided by financing activities totaled $1.6 million in 1997, $2.9 million in 1998, while cash used by financing activities was $4.2 million in 1999. In 1997 and 1998, new borrowings exceeded payments on existing debt by $1.2 million and $6.8 million. This increase in debt was required because cash flow from operating activities was not adequate to cover the high level of investing activities taking place. In 1999, payments on outstanding loans exceeded new borrowings by $4.2 million.
Our sources of available funds as of June 30, 2000 were comprised of $1.9 million in cash and cash equivalents and a revolving credit agreement with a commercial bank with aggregate borrowing capacity of $2.5 million. We also have term notes payable aggregating $5.1 million at variable interest rates as of June 30, 2000.
In July 2000, we entered into a term loan credit agreement with U.S. Bank National Association. This term loan consists of a $1.7 million note which is payable beginning September 1, 2000. This loan also requires monthly payments of principal and interest of $12,694. Interest on the outstanding debt is equal to a floating rate equal to the sum of the adjusted Eurodollar rate plus 1.9%. The outstanding principal together with all unpaid interest is due on August 1, 2005.
We believe that our cash and cash equivalents, cash flow from operations and available credit facilities, together with the proceeds of this offering, will be sufficient to meet our working capital and investing requirements for the next twelve months. However, our future growth, including potential acquisitions, may require additional funding, and from time to time we may need to raise capital through additional equity or debt financing. If we were unable to obtain this additional funding, we might have to curtail our expansion or acquisition plans. There can be no assurance that any such financing would be available to us on commercially acceptable terms.
Recent Accounting Pronouncements
SFAS No. 133, Accounting for Derivative Instruments and Hedging Activities, was issued in June 1998 and establishes accounting and reporting standards for derivative instruments. The statement requires recognition of all derivatives as either assets or liabilities in the balance sheet measured at fair value and will be effective in fiscal year 2001. We do not anticipate that the adoption of SFAS No. 133 will have a significant impact on our financial position or results of operations.
Quantitative and Qualitative Disclosure About Market Risk
Market risk is the risk that we will incur losses due to adverse changes in interest rates, foreign currency exchange rates, and raw materials availability and pricing.
30
We sell products and services in currencies other than the U.S. dollar. Consequently, we are subject to profitability risk arising from exchange rate movements. In 1997, 1998, 1999, and the six months ended June 30, 2000, we did not engage in any foreign exchange contracts or hedging activities. As of June 30, 2000, the foreign currencies to which we had the most significant exchange rate exposure were the Japanese yen, the European euro, and the British pound. We do not use derivative financial instruments for trading purposes.
We had $7.4 million in long-term debt as of June 30, 2000. Of this amount, $7.3 million was held at variable interest rates. We have cash flow exposure on our committed and uncommitted revolving note payable and variable long-term debt due to variable Eurodollar and LIBOR rate pricing. As of June 30, 2000, a 1% change in the Eurodollar rate or LIBOR rate would not materially increase or decrease interest expense or cash flows.
We had approximately $0.1 million in long-term debt at a fixed interest rate of 7.5% per annum. This debt is subject to interest rate risk and will increase or decrease in value if market interest rates change.
We depend on various materials, including tantalum, pyrolytic boron nitride, molybdenum and stainless steel, for the manufacture of our products. We do not have long-term supply agreements with our raw material suppliers and purchase such materials on a purchase order basis. Our gross margins are sensitive to fluctuations in the prices of these raw materials. In particular, recent increases in the spot price for tantalum and pyrolytic boron nitride may have a negative impact on our future gross margins.
31
Our Business
We are a leading provider of epitaxial equipment and related components used to produce compound semiconductors for the fiber optic and wireless markets, as well as for other consumer applications. Our product line includes epitaxial equipment and related components used in both high and low volume commercial production, and research and development. For 14 years, we have offered products and services designed to cost-effectively meet the increasingly demanding production requirements of the global high-performance communications infrastructure. We sell our products to compound semiconductor manufacturers, epiwafer suppliers, as well as research and academic institutions. In 1999, our major commercial customers in the fiber optic market included Bandwidth9, Inc., JDS Uniphase Corporation, Lucent Technologies Inc., Mitsubishi Chemical Corporation, and NEC Corporation, and in the wireless market included Agilent Technologies, Inc., IQE plc, Raytheon Company, RF Micro Devices, Inc. and Sanders, a Lockheed Martin Company. International sales represented approximately 32% of our sales in 1997, 35% in 1998, 40% in 1999 and 38% in the six months ended June 30, 2000.
Industry Overview
The growing demand for information and connectivity is driving the continued rapid expansion of the global communications infrastructure, including high-performance fiber optic and wireless networks. Compound semiconductors are key components of these networks and the products that rely on these networks. Fiber optic and current generations of wireless applications require greater performance and functionality than silicon semiconductors can provide, and as a result, compound semiconductors have emerged as a key enabling technology to meet the needs of these systems. Compound semiconductors are core components of a wide variety of communications applications, including fiber optic modules and subsystems, mobile phones, wireless networks and satellites. Compound semiconductors are also used in several growing consumer applications, including optical data storage, low cost lighting and signage and global positioning systems.
Compound semiconductors are composed of two or more elemental materials usually consisting of a metal, such as gallium, aluminum or indium, and a non-metal, such as arsenic, phosphorous or nitrogen. Compound semiconductors have intrinsic physical properties that enable electrons to move approximately five times faster than through silicon semiconductors, allowing these semiconductors to operate more efficiently and at higher speeds. In addition, unlike silicon semiconductors, compound semiconductors have optoelectronic properties that enable them to emit light, which are essential for fiber optic applications. Other key advantages include lower power consumption and reduced signal noise and distortion, which are critical to optimal performance of current generations of wireless technologies.
The compound semiconductor market was approximately $11.0 billion in 1999 and is expected to grow 24% to approximately $13.6 billion in 2000, according to Strategies Unlimited. Communications applications account for approximately 50% of the overall compound semiconductor market and are expected to be the fastest growing segment going forward. This growth is expected to be driven by the anticipated continued high demand for fiber optic and wireless communications.
Fiber optic technology improves the capacity and speed of transferring data through a network. Compound semiconductors enable various key components of fiber optic networks, such as the source lasers, pump lasers, multiplexers and demultiplexers used in dense wavelength division multiplexing, or DWDM. According to Strategies Unlimited, sales of compound semiconductors for fiber optic communications in 1999 were valued at approximately $2.6 billion and are expected to grow by more
32
Increasing demand for connectivity is driving the growth in wireless networks and increasing usage of mobile phones. According to Global Mobile, the number of wireless phone subscribers is expected to grow from approximately 478 million worldwide in 1999 to nearly one billion by 2002, representing a compound annual growth rate of 28%. Mobile phones are expected to be a significant growth driver for the compound semiconductor market, given that a typical mobile phone uses a number of compound semiconductor devices. The advent of multi-mode high bandwidth phones further increases the demand for compound semiconductors due to their higher performance requirements.
Compound semiconductors are also used in several consumer-oriented and other applications, such as optical data storage for digital versatile disks (DVDs) and compact disks (CDs), high brightness LEDs for lighting and signage, sensors used in automotive anti-lock brake systems, automotive collision avoidance radar and global positioning systems.
The following table highlights some of the primary applications, devices, key technological advantages and benefits of compound semiconductors:
| Technological | ||||||||
| Market | Applications | Devices/Components | Advantages | End-User Benefits | ||||
| Fiber optic communications |
• DWDM • Fiber channel • Gigabit ethernet • Wide area networks (WANs) • Local area networks (LANs) |
• Pump lasers • Vertical cavity surface emitting lasers (VCSELs) • Edge emitting lasers • Optical amplifiers • Multiplexers/ demultiplexers • Laser drivers/amplifiers • Clock data recovery • Photo detectors |
• Light emitting capabilities • Higher speed and frequencies |
• Increased bandwidth • Increased network capacity • Increased data transmission speeds |
||||
| Wireless communications |
• Mobile telephones • Point-to-multipoint fixed wireless access • Wireless LANs • Personal digital assistants (PDAs) • Bluetooth • Pagers |
• Power amplifiers • Low noise amplifiers • Receivers • Switches • Converters |
• Lower power consumption • Lower heat generation • Higher speed and frequencies • Improved signal to noise ratio |
• Longer battery life • Signal clarity • Increased network capacity • Reduced size • Remote Internet access |
||||
| Consumer applications |
• DVDs • CDs • Cellular/PCS display panels • Flat panel displays • Illuminated billboards and signs • Global Positioning Systems • Antilock brake systems |
• Laser diodes • High brightness LEDs • Magnetic sensors • Receivers |
• Light emitting capabilities • Higher speed and frequencies • Magnetic sensitivity |
• Display clarity • Increased data storage capacity • Low cost lighting |
||||
A full line of fabrication equipment and processes is required to produce compound semiconductor devices. Fabrication equipment includes epitaxial, inspection, photolithography, etching, deposition, cleaning, testing and packaging equipment. For the majority of compound semiconductors, epitaxy is the critical first step of the fabrication process, whereby very thin crystalline layers consisting of various elemental materials are precisely deposited on a substrate to create a compound semiconductor. The unique performance characteristics of compound semiconduc-
33
The two most common epitaxial technologies used to manufacture compound semiconductors are molecular beam epitaxy (MBE) and metal organic chemical vapor deposition (MOCVD). While some compound semiconductor applications favor either MBE or MOCVD, either technology can be used for most applications. Due to the large demand for higher performance compound semiconductor devices, a cost-effective, higher volume production solution has been needed to increase throughput, uptime and yield, while maintaining a high level of precision.
The Applied Epi Solution
We are a leading provider of epitaxial equipment and related components used to produce compound semiconductors for the fiber optic and wireless markets, as well as for other consumer applications. For 14 years, we have offered products and services designed to cost-effectively meet the increasingly demanding production requirements of the global high-performance communications infrastructure. The primary aspects of our solution consist of:
Technology Innovator
We have been an innovator in the compound semiconductor equipment and component industry since our inception, capitalizing on our advanced materials expertise, our comprehensive understanding of ultra high vacuum technologies and our specialized ability to manipulate refractory metals. Our product line represents a number of industry-firsts, including the following key components of epitaxial equipment:
| • | material-specific effusion cells, handling the unique characteristics of specific groups of elements used in compound semiconductors; | |
| • | valved crackers, leading to increased reliability and control during growth of the critical epitaxial layers; | |
| • | SUMO effusion cells, designed to significantly increase equipment capacity and uptime; and | |
| • | automated UHV multiwafer handling cluster tools, enabling high volume production while maintaining high purity conditions. |
Comprehensive Product Offering
Throughout our history, we have continually improved our products and enhanced our product line to meet our customers’ increasingly demanding requirements. We believe that we offer the broadest product selection of epitaxial equipment and components for the compound semiconductor industry. For high volume production applications, we provide manufacturing equipment featuring an automated UHV multiwafer handling cluster tool. For low volume production and research applications, we provide highly reliable, configurable systems that are capable of addressing a wide variety of applications. We are also a leading supplier of key components to the MBE equipment market, including effusion cells, control hardware and electronics, process control software, spare parts and consumables.
34
High Volume Production MBE Systems with Low Cost of Ownership
The main factors determining the cost of ownership for compound semiconductor equipment are throughput, uptime, yield, initial capital investment and operating costs. These key factors impact the cost of ownership for our customers, influence their purchase decisions and ultimately affect their success in the marketplace. Our GEN2000 is the world’s first high volume production MBE system incorporating cluster tool architecture, designed to provide our customers with increased productivity and lower cost of ownership. The GEN2000’s advances in productivity stem from its ability to process seven 6” wafers (7x6”) simultaneously, currently the largest capacity in the world, and its use of an automated UHV multiwafer handling cluster tool. Also, the GEN2000 leverages our advanced SUMO effusion cells and valved cracker technology to maximize uptime. By combining the advantages of the GEN2000’s cluster tool, 7x6” capacity and advanced effusion cell technology, the GEN2000 is designed to reduce manufacturing cost per wafer by approximately 50% over currently available systems.
Leading Provider of Critical Components for the MBE Equipment Market
The MBE equipment market has come to rely on our comprehensive portfolio of components including effusion cells, control hardware and electronics, process control software and consumables. We possess proprietary technology for the design and manufacture of numerous components that are critical to the operation of an MBE system, such as effusion cells. Customers who purchase MBE systems from other manufacturers often request that our effusion cells and other components be used in the purchased system. We estimate that more than 70% of the world’s compound semiconductor manufacturers using MBE systems use our effusion cells on their equipment. We believe that the reliability, capacity and serviceability of our effusion cells and other components give us substantial competitive advantages in the marketplace.
Applied Epi’s Strategy
Our goal is to become the leading supplier of integrated equipment and related process solutions to compound semiconductor manufacturers for use throughout their fabrication line. Key elements of our strategy are as follows:
Further Penetrate High Volume MBE Equipment Markets
Since its introduction in January 2000, we have seen strong demand for our high volume production MBE system, the GEN2000. Both the GEN2000 and our other high volume production system, the GEN200, offer compound semiconductor manufacturers many benefits, including high precision, high throughput and low cost of ownership. We intend to continue to leverage our strong presence in the MBE component and low volume production and research equipment markets, as well as our established global customer base to further penetrate the high volume production MBE equipment market.
Leverage Current Technology and Relationships to Penetrate MOCVD Market
In order to provide our customers a choice of epitaxial technology platforms, we are leveraging our core competencies in MBE technology to develop production MOCVD equipment. These core competencies include our expertise in substrate heating control and process automation, as well as our specialized ability to manipulate refractory metals. We have chosen an established architecture for our production MOCVD equipment and expect to introduce our first production MOCVD tool in the
35
Expand Product Offering to Provide Complete Fabrication Line Solutions
We believe compound semiconductor manufacturers are seeking suppliers who provide a wide range of products that address multiple aspects of the fabrication line, including integrated equipment and related process solutions. To reinforce our position in, and expand beyond, the epitaxial equipment market, we intend to pursue acquisitions to become the leading supplier of complete fabrication line solutions to compound semiconductor manufacturers. We also intend to pursue internal development, strategic partnerships and alliances. We currently have no agreements or commitments to acquire any business.
Enhance Customer Relationships
We have established close working relationships with leading compound semiconductor manufacturers. We intend to enhance those relationships by opening a Process Integration Center (PIC) in 2001, where we will work together with our customers to test and develop new equipment and processes. Initially, the PIC will be focused on epitaxial equipment and processes. Ultimately, we expect the PIC to house the equipment and expertise necessary for our customers to develop entire integrated equipment and process solutions for various aspects of their compound semiconductor fabrication line. We believe the PIC will allow our customers to qualify new material and equipment technologies prior to shipment, while reinforcing our relationship with them. Additionally, we intend to continue to expand our worldwide service network with additional personnel, as well as to offer additional service and support packages specifically designed for our high volume manufacturing customers.
Extend Technology Leadership
We have a history of innovations, which has provided us with competitive advantages in the marketplace. We will continue to invest in research and development in those areas that we deem strategic and those having the highest growth potential, thereby maintaining our technological leadership position and further increasing the barriers to entry in our industry. We also intend to expand our intellectual property portfolio by pursuing internal development, joint ventures and acquisitions. We will continue to use our comprehensive knowledge of compound semiconductor fabrication line operations to provide for our customers’ technology requirements.
Technology
Epitaxial Technologies
Epitaxy is the critical enabling first step in the production of most compound semiconductors, ultimately determining device functionality and performance. Epitaxy is the process of depositing, or growing, atomically thin crystal layers of typically dissimilar elemental materials onto a substrate to produce a compound semiconductor. Each crystal layer is known as an epitaxial layer, or epilayer. After the epilayers are grown on a substrate, it is known as an epiwafer.
A number of elemental materials are used to grow the epilayers, such as gallium, arsenic, indium, phosphorous, nitrogen, silicon, germanium, antimony, aluminum and beryllium. As the layers are grown, impurities known as “dopants” are selectively incorporated into the epilayer, modifying the electronic and optoelectronic properties of the crystal. Material composition, dopant concentration,
36
The two most common epitaxial technologies used to manufacture compound semiconductors are MBE and MOCVD. While some compound semiconductor applications favor either MBE or MOCVD, either technology can be used for most applications. An important part of our strategy is to offer our customers the choice of either technology platform to fit their specific performance criteria for compound semiconductors.
Liquid Phase Epitaxy (LPE) and Vapor Phase Epitaxy (VPE) are two additional epitaxial technologies with limited commercial use because of their inability to produce thin, uniform layers. Historically, LPE and VPE have been associated with the production of conventional, low-brightness LEDs. In addition, a small percentage of total compound semiconductor revenues today come from ion implant technology, which is not an epitaxial process.
Molecular Beam Epitaxy
MBE is an epitaxial deposition technique involving the reaction of one or more thermal beams of atoms or molecules with a heated crystalline wafer substrate under ultra high vacuum conditions. The thermal beams are made up of the constituent elemental materials that will be used to form the compound semiconductor. The most common materials used today are gallium, arsenic and aluminum used to produce gallium arsenide and aluminum gallium arsenide.
The molecular beams are typically generated by thermally evaporating elemental source material in a component called an effusion cell. Effusion cells, and the high purity source material put in them, are the most critical components to successful operation of an MBE system. Source materials are typically solid, but may also be introduced in the form of a gas. During operation, the effusion cells are directed at the wafer substrate so that the molecular beams will impinge upon the wafer, depositing an epilayer. Precise control of the growth conditions is required when creating a compound semiconductor. Optimum uniformity is achieved by employing exacting measures during design and manufacturing of the MBE growth chamber and effusion cells, and by rotating the substrate during the growth process. Layer thickness and composition are precisely controlled by:
| • | varying the temperature of the effusion cells to control beam output; | |
| • | starting and stopping the molecular beams with shutters and valves; and | |
| • | adjusting the substrate temperature. |
37
The following chart illustrates the basic MBE growth process:
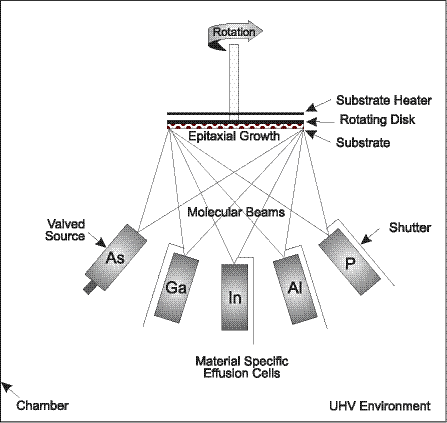
In addition to the typical MBE technology described above, variants of MBE technology include Chemical Beam Epitaxy (CBE), Gas Source Molecular Beam Epitaxy (GSMBE) and Metal Organic Molecular Beam Epitaxy (MOMBE).
Metal Organic Chemical Vapor Deposition
MOCVD is an epitaxial deposition technique involving the transportation of reactive gas compounds via a carrier gas to a heated rotating substrate. This is different than MBE in that the gases used in MOCVD are chemical compounds delivered via pipes and valves, as opposed to atoms in elemental form directed at the substrate from an effusion cell. The chemical compounds are combinations of metals and organic molecules, hence the name “metal-organic.” MOCVD uses a variety of chemical compounds in order to provide each constituent component of a compound semiconductor. An example is trimethyl gallium, a combination of an organic molecule and the metal gallium. The metal component will stick to the substrate in the presence of heat forming a part of the compound semiconductor, while the organic component will flow out of the chamber. Other chemical compounds include trimethyl indium as a source of indium, arsine as a source of arsenic and phosphine as a source of phosphorous.
MOCVD crystal growth takes place inside a chamber. In a typical system, the wafer substrate is mounted onto a rotating flat surface or a disk and is heated to high temperatures during the epitaxial growth process. Various gases flow from tanks through pipes and valves that are used to select different gas mixtures which then flow into the reaction chamber. A uniform flow of gas, usually hydrogen, is maintained in the chamber. This gas flows directly onto the rotating disk and wafer, and is then exhausted. Reactive metal organic gas compounds are added to this flow. These gases combine at the hot surface of the rotating disk and react to form the epilayers. Layer composition and thickness are controlled by switching between gases and valving the gases on or off. The rate of deposition is governed by chemical reactions at the substrate. Therefore, layer thickness and
38
The following chart illustrates the basic MOCVD growth process:
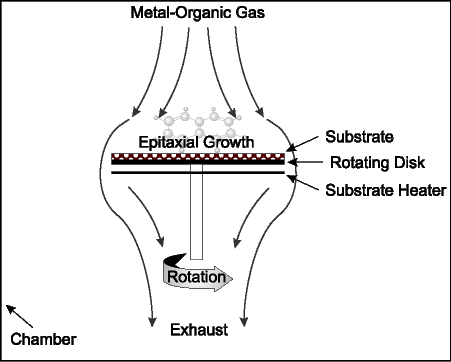
MOCVD is also known as Metal Organic Vapor Phase Epitaxy (MOVPE) and Organo-Metallic Vapor Phase Epitaxy (OMVPE).
Products
We began manufacturing MBE equipment and related components in 1986 with our introduction of material specific effusion cells. Since then, we have continually improved our products to address specific customer needs such as material quality, throughput, uptime, yield, initial capital investment and operating costs. These key factors impact the cost of ownership for our customers and ultimately affect their success in the marketplace.
We believe we have the broadest product offering of epitaxial equipment and components for the compound semiconductor industry. We divide our products into four main categories: high volume production MBE systems, low volume production and research MBE systems, production MOCVD systems and performance products.
High Volume Production MBE Systems
In the third quarter of 2000, we shipped our first GEN2000 production MBE system, which has been designed to provide our customers with increased productivity and lower cost of ownership. The GEN2000’s advances in productivity stem from its ability to process seven 6” wafers (7x6”) simultaneously, currently the largest capacity in the world, and its utilization of an automated UHV multiwafer handling cluster tool. In addition, the GEN2000 leverages our advanced SUMO effusion cells and valved cracker technology to maximize uptime.
The GEN2000’s flexible modular design permits different configurations to meet specific customer requirements. A typical configuration is illustrated in the diagram below, consisting of a
39
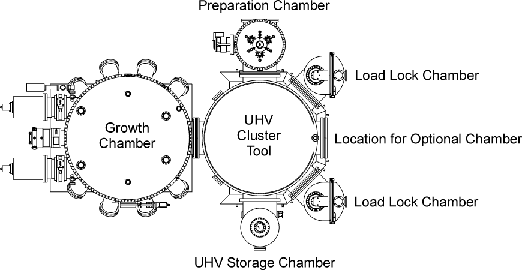
The load lock allows wafers to be brought into and taken out of the system while maintaining vacuum conditions in the other chambers. The load lock chambers accommodate eight platens (wafer carriers) per chamber. With each platen holding seven 6” wafers (7x6”), a chamber can hold fifty-six 6” wafers at once, minimizing the need to reload the system. Additional storage capacity is achieved with the UHV storage chamber which can accommodate ten platens. The UHV cluster tool prevents contaminants from reaching the MBE growth chamber which may have been introduced in the load lock. The MBE growth chamber is where the actual epilayer growth takes place. In addition, the cluster tool can accommodate an optional chamber, such as another MBE growth chamber, storage chamber or an inspection and test chamber.
The GEN2000’s automated UHV multiwafer handling cluster tool, modeled after proven silicon architecture, is a first for the compound semiconductor industry. The cluster tool is a precision robotic, multiwafer handling system designed specifically to withstand the demanding UHV conditions of MBE. Unlike conventional cluster tools found in the silicon industry, the GEN2000 cluster tool can withstand external temperatures up to 200 degrees Celsius (approximately 392 degrees Fahrenheit) and operate without lubricants of any kind in order to avoid contaminants in the UHV chamber. The demanding operating environment required for producing compound semiconductors is very different than that of the silicon semiconductor industry and presents significant engineering challenges for equipment developers. Robotic multiwafer handling is becoming essential for high volume applications providing higher throughput than manual wafer handling.
Our GEN200 production MBE system leverages the same advanced technology as the GEN2000, including the UHV multiwafer handling cluster tool and high capacity effusion source technology. Both systems provide a high level of precision control, while providing our customers with a choice of the level of capacity and throughput desired.
Low Volume Production and Research MBE Systems
We offer a complete line of low volume production and research MBE systems. Our most popular system in this category, the Modular GENII, has an installed base of over 200 systems. The
40
Our other low volume production and research MBE systems include: the GENIII which can be configured for epitaxial growth on either a single 4” wafer or three 2” wafers, the 930 system for epitaxial growth on a single 3” wafer, and the 620 system for epitaxial growth on a single 2” wafer.
Production MOCVD Systems
We are in the process of developing our production MOCVD system based on an established reactor architecture. The fundamental design of this architecture has been proven in the industry for the production of compound semiconductor devices for optical and electronics applications. Many of our core competencies, such as our expertise in substrate heating control and process automation, as well as our specialized ability to manipulate refractory metals assist us in the development of MOCVD technology. We expect that our first MOCVD systems will be introduced in the second half of 2001.
Performance Products
Our performance products are components used in compound semiconductor epitaxial manufacturing equipment, and include effusion cells, control hardware and electronics, software and spare parts. We are a leading supplier of key components to the MBE equipment market, and estimate that more than 70% of the world’s compound semiconductor manufacturers using MBE systems use our effusion cells on their equipment.
We were the first to develop material specific effusion cells to suit the unique characteristics of specific groups of elements used in compound semiconductors. For example, our arsenic crackers are designed specifically for supplying thermally decomposed, or cracked, arsenic during the MBE deposition process for compounds like gallium arsenide. One of our most popular effusion cell is the arsenic valved cracker, which not only cracks arsenic molecules, but regulates the beam output during the MBE deposition process with a patented valve mechanism. Valved effusion cells provide the important capability of controlling the molecular beam output with a high degree of precision.
The demanding environment of multiwafer production MBE systems requires robust effusion cells with large capacities to maximize system uptime. Another example of our innovation in this field is the development of an advanced cell technology for gallium or indium, known as SUMO. SUMO technology significantly increases the total capacity and uptime of a production MBE system. The patented SUMO technology incorporates proprietary materials and mechanical designs to form a specifically shaped crucible component that holds twice the material of prior technologies. Because of our SUMO cells’ increased capacity, which enables greater system uptime, they have become the industry standard for high volume commercial applications throughout the world.
41
The following table outlines the breadth of our effusion cell product line:
| Effusion Cell Type | Materials Supported | |
| Group III SUMO sources | Gallium (Ga), Indium (In), Aluminum (Al) | |
| Low temperature SUMO sources | Lithium (Li), Potassium (K), Sodium (Na), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Zinc (Zn), Cadmium (Cd), Thallium (Th), Lead (Pb), Bismuth (Bi), Europium (Eu), Ytterbium (Yb), Cadmium Selenide (CdSe), Cadmium Sulfide (CdS), Lead Selenide (PbSe), Lead Telluride (PbTe), Tin Telluride (SnTe), Zinc Sulfide (ZnS), ZINC Selenide (ZnSe) | |
| Dopant sources | Beryllium (Be), Carbon (C), Nitrogen (N), Silicon (Si) | |
| Standard or modified filament sources | Manganese (Mn), Silver (Ag), Gold (Au), Germanium (Ge), Tin (Sn), Dysprosium (Dy), Erbium (Er) | |
| RF plasma sources | Nitrogen (N), Oxygen (O), Hydrogen (H), Methane (CH4) | |
| Special valved sources | Mercury (Hg), Zinc Chloride (ZnCl) | |
| High temperature sources | Scandium (Sc), Yttrium (Y), Lanthanum (La), Titanium (Ti), Chromium (Cr), Iron (Fe), Cobalt (Co), Nickel (Ni), Palladium (Pd), Platinum (Pt), Copper (Cu), Boron (B), Praseodymium (Pr), Gadolinium (Gd), Lutetium (Lu) | |
| Valved crackers | Arsenic (As), Phosphorous (P), Selenium (Se), Sulfur (S) | |
| Standard crackers | Arsenic (As), Antimony (Sb), Sulfur (S), Selenium (Se) | |
| High temperature gas crackers | Arsine (AsH3), Phosphine (PH3), Hydrogen (H) | |
| Valved crackers for corrosive materials | Antimony (Sb), Tellurium (Te), Cadmium Telluride (CdTe) | |
| Low temperature gas injectors | Ammonia (NH3), Carbon Tetrabromide (CBr4), Metalorganics (TEGa, TEIn), Disilane (Si2H6) | |
We also sell control hardware and software for the operation of our performance products and systems. These products include modular temperature controllers, power supplies and automation equipment. In addition, we offer our Molly line of software to facilitate automated process control. Furthermore, we supply a wide range of spare parts and other consumable items to support our installed base of components and systems.
Customers
We have a diverse global customer base. Our primary types of customers are compound semiconductor manufacturers, epiwafer suppliers, and research and academic institutions. No single customer accounted for more than 10% of our sales in 1998 or 1999. Our top ten customers accounted for approximately 38% of our sales in 1998 and approximately 46% of our sales in 1999.
42
The following table sets forth our top ten fiber optic and other optoelectronics, wireless and academic and research customers in 1999:
| Fiber Optic and | ||||
| other Optoelectronics | Wireless | Academic and Research | ||
| ADC Telecommunications, Inc | Agilent Technologies, Inc. | Fraunhofer IAF | ||
| Bandwidth9, Inc. | Alpha Industries, Inc. | Hong Kong Polytechnic University | ||
| Cielo Communications, Inc | HRL Laboratories, LLC | Laboratorio Nazionale TASC-INFM | ||
| JDS Uniphase Corporation | IQE plc | Oxxel Oxide Electronics Technology GmbH | ||
| Lucent Technologies Inc. | Motorola, Inc. | State University at Albany, New York | ||
| Mitsubishi Chemical Corporation | Picogiga SA | University of California at Santa Barbara | ||
| NEC Corporation | Procomp Informatics, Ltd. | University of Michigan | ||
| OKI Electric Industry Co., Ltd | Raytheon Company | University Of Tampere | ||
| Sharp Electronics Corporation | RF Micro Devices, Inc. | Walter-Schottky-Institute | ||
| Thomson CSF — Systems Canada | Sanders, a Lockheed Martin Company | Wright Patterson Air Force Base | ||
Sales and Marketing
We market, sell and distribute our products through a worldwide network composed of our direct sales force and third-party sales representatives. In the United States, we divide our direct sales efforts geographically into the west coast, central and east coast regions, with offices in Boston, Massachusetts; Santa Clara, California; and our headquarters in St. Paul, Minnesota. Our European sales effort is managed by our direct sales force from our office in Greater Manchester, United Kingdom. We also have a sales representative in France. Our Asia-Pacific sales effort is managed by our Asia-Pacific sales manager based at our headquarters in St. Paul, Minnesota. Our Asia-Pacific sales manager also manages our sales representatives in that area, including our sales representatives in Taiwan, Singapore, China, India and Japan.
An integral part of our sales and marketing effort is the involvement of our product managers who supply in-depth product knowledge and technical support to our customers, account managers and sales agents. Our sales agents, account managers, and product managers work together closely to market products, develop relationships with prospective customers and close sales transactions. The sales process associated with the initial purchase of our systems is typically complex and lengthy. The sales process for subsequent purchases by the same customer is generally shorter.
We also have a business development team which is responsible for identifying market trends and new opportunities that will impact our sales effort. The business development team works with the sales and product management team to incorporate this information into our sales strategies. Our product advertising efforts are focused on tradeshow activities throughout the world, direct mail, product brochures and advertisements in industry trade publications.
International sales accounted for approximately 32% of our sales in 1997, 35% in 1998, 40% in 1999, and 38% in the six months ended June 30, 2000.
We support our sales and marketing efforts with a global service and support network with the goal of providing our customers with complete, accurate, competent and timely responses to all inquiries. Our service and support network is operated from our offices located in the United States and United Kingdom. In addition, our sales representative and support arrangements in Japan, Taiwan, Korea, China, Singapore, India and France are an integral part of our service and support
43
We offer a one-year warranty on our low volume production, research and high volume production MBE systems. We currently offer a two-year warranty on our effusion cell products.
Research and Development
Our industry is characterized by rapid change and development. We believe that our research and development programs are critical to the successful implementation of our growth strategy. We intend to devote additional financial and human resources to our research and development efforts, focusing primarily on:
| • | continuing innovation of MBE equipment and technologies; | |
| • | developing MOCVD equipment and technologies; | |
| • | developing the Process Integration Center; and | |
| • | improving the performance of our existing products. |
We intend to open our Process Integration Center (PIC) in 2001. The PIC will enable us to work with our customers to develop and design the equipment and related processes necessary to meet the specific compound semiconductor requirements of our customers.
In an effort to improve our compound semiconductor technologies, we have established relationships and ties with leading universities and corporate research laboratories in the field of compound semiconductors, such as the University of California at Santa Barbara; University of Texas, Austin; University of Tampere, Finland; University of Glasgow, United Kingdom; Hughes Research Laboratories and Agilent Technologies, Inc.
Manufacturing
Our systems and components are assembled and tested at our manufacturing facilities in St. Paul, Minnesota. We have a sophisticated in-house machining capability which allows us to manufacture many of the specialized tools, dies and parts required for the assembly of our equipment and related components. We also have a specialized ability to manipulate refractory metals, which is a crucial element of the assembly process due to the extreme temperature variations between various chambers of our MBE systems.
Depending on the number of options selected by a customer, generally five to six months of manufacturing and assembly time is required for the manufacture of one of our systems. An additional one to four months is usually required to install, test and obtain customer acceptance.
We use subcontractors for the manufacture of various parts and electronic assemblies for our systems and related components. We also use suppliers for off-the-shelf products such as pumps, ultra high vacuum components and electronics and for raw materials, such as tantalum, tungsten and pyrolytic boron nitride.
44
Our quality assurance department is staffed with technicians who supervise the assembly process and implement our quality assurance program. Additionally, all of our products undergo rigorous testing before shipment.
Intellectual Property and Other Proprietary Rights
Our success and ability to compete are dependent to a significant degree on our proprietary technology. We rely on a combination of patent, copyright, trademark and trade secret laws, as well as confidentiality procedures, contractual provisions and other similar measures to establish and protect our intellectual property and proprietary rights.
We actively pursue a program of patent applications to seek protection of technologically sensitive features of our technologies. As of September 25, 2000, we had 12 U.S. patents issued, two U.S. patent applications pending and other applications that we are currently preparing. We have also filed counterpart applications for some of our patents in Europe and Japan. We cannot assure you that these patents will be issued, or that even if issued, these or any of our other patents will adequately protect our technology or processes or otherwise result in commercial or competitive advantages to us. In addition, we have the exclusive rights to three additional patents under license agreements with the University of Texas which relate to our effusion cell products, including valved crackers. If we are unable to pay royalties under these license agreements, our rights to use those patents and manufacture these products could be harmed.
We seek to avoid disclosure of our trade secrets through a number of means, including requiring substantially all of our employees, consultants and other persons with access to our proprietary information, to execute confidentiality agreements with us and by restricting access to our proprietary information. In addition, we seek to protect our documentation, software and other written materials under trade secret and copyright laws, which afford only limited protection.
SUMO and UNIBLOCK are our registered trademarks. Applied Epi, GEN2000, GEN200, GENII, GENIII, Molly and certain other names not included in this prospectus are our trademarks. Registrations of the Applied Epi name and logo as well as other names used in our business are pending. Trademark applications are subject to consideration by the U.S. Patent and Trademark Office, may be opposed by private parties, and may not be issued. We cannot assure you that any of our proprietary rights will be viable or of value in the future.
Despite our efforts to protect our intellectual property and other proprietary rights, existing patent, copyright, trademark and trade secret laws afford only limited protection. In addition, the laws of some foreign countries do not protect our proprietary rights to the same extent as do the laws of the United States. Attempts may be made to copy or reverse engineer aspects of our products or to obtain and use information that we regard as proprietary. Accordingly, we may not be able to prevent misappropriation of our technology or deter others from developing similar technology. Furthermore, policing the unauthorized use of our intellectual property and other proprietary rights is difficult. Litigation may be necessary in the future to enforce our intellectual property rights or to determine the validity and scope of the proprietary rights of others. This litigation could result in substantial costs and diversion of resources and could significantly harm our business.
Backlog
As of August 31, 2000, we had an order backlog of $22.6 million, scheduled to be shipped through April 2001. Our backlog has increased during the third quarter of 2000 primarily due to orders for the GEN2000. We include in our backlog only customer purchase orders that have been accepted by us and for which shipment dates have been assigned within the next 12 months. All
45
Competition
The epitaxial equipment and related components markets are highly competitive. We believe that the primary competitive factors in the MBE and MOCVD equipment markets are technical product performance, cost of ownership, service and support, product quality and reliability. We believe we compete favorably based on these factors and are not aware of any single competitor who offers a comparable breadth of products in our served markets. In addition, we believe we have a leading position in the markets for low volume production and research MBE equipment, as well as for related MBE components.
Many of our competitors have significantly more experience in developing, manufacturing and marketing high volume production MBE and MOCVD systems. Our two largest competitors in the MBE equipment and component market are VG Semicon, a division of Thermo Electron, based in the United Kingdom, and Riber SA, based in France. Other competitors in the MBE equipment markets consist of a number of smaller companies. Our three largest competitors in the MOCVD equipment market are Aixtron AG, based in Germany, Emcore Corporation, based in New Jersey, and Nippon-Sanso K.K., based in Japan.
Some of our competitors and potential competitors have longer operating histories and significantly greater financial resources than we do. In addition, many of these companies may have more technical and marketing personnel, and more established customer support organizations than we do. It is also possible that new competitors and alliances among our competitors may emerge and rapidly acquire significant market share. Our competitors may be able to respond more quickly to new or emerging technologies and changes in customer requirements than we can.
Facilities
Our principal executive offices are located in St. Paul, Minnesota, where we own approximately 75,000 square feet under a mortgage which matures in December 2019. Approximately 45,000 square feet of our headquarters is dedicated to our manufacturing and assembly operations. We also lease office space in Santa Clara, California; Boston, Massachusetts and Greater Manchester, United Kingdom.
We own additional land adjacent to our St. Paul, Minnesota facility and plan to commence building of our expanded facility in the fourth quarter of 2000. The new building will be approximately 43,000 square feet, will be used for manufacturing and will be built using proceeds from this offering. We also own additional adjacent land, reserved for future expansion.
Employees
As of August 31, 2000, we had a total of 112 employees, 26 of which were engineers. None of our employees are subject to a collective bargaining agreement and we believe that our relations with our employees are good.
46
Regulations
We are subject to various federal, state and local environmental, health and safety laws and regulations. We maintain small amounts of acids and other chemical agents for our manufacturing and service operations. In addition, we provide chemical decontamination and cleaning services on our customers’ MBE systems and related components that involve the handling, and in some cases disposal, of arsenic or phosphorus-based compounds and other hazardous materials. Operation and development of epitaxial equipment could result in the release of hazardous materials. In addition, use of MOCVD systems involves larger amounts of hazardous materials than with MBE technology. If our control systems and safety procedures are unsuccessful in preventing release of these materials into the environment or exposure of our personnel to occupational hazards, we may be subject to liability under environmental, health and safety laws and could incur significant expenses. We believe that we are, and have been in the past, in compliance with all such laws and regulations.
Export of our products to certain destinations, such as China and Taiwan, may require an export license from U.S. export control authorities. To date, we have not experienced difficulties obtaining export licenses, but a delay or a rejection of an export license application may affect our revenues.
Legal Proceedings
We are not currently involved in any legal proceedings which we expect could materially harm our business, financial condition or results of operations either individually or in the aggregate. Early this year, we filed a lawsuit against Riber SA and Riber Inc. alleging infringement of a patent licensed to us covering certain effusion cells. Riber then filed a counterclaim alleging that we infringed two of their patents covering certain phosphorous effusion cells. In September 2000, we entered into a settlement agreement with Riber which terminated the lawsuit and requires each of us to cross-license and pay the other a royalty based on sales of equipment covered by each of our respective patents subject to the lawsuit. We do not expect that any royalty income we receive or royalty expenses we incur under such license agreements to have a material impact on our financial results.
47
Executive Officers and Directors
The following table sets forth certain information with respect to our executive officers and directors.
| Name | Age | Position(s) | ||||
| Paul E. Colombo | 40 | Founder and Chairman | ||||
| David G. Reamer | 35 | President, Chief Executive Officer and Director | ||||
| Marlin A. Braun | 39 | Vice President of Strategic Marketing and Business Development | ||||
| Brett D. Heffes | 33 | Chief Financial Officer and Treasurer | ||||
| Frank C. Kraemer | 46 | Chief Operating Officer, Secretary and Director | ||||
| William J. Cadogan(1)(2) | 52 | Director | ||||
| Noel P. Rahn(1)(2) | 61 | Director | ||||
| (1) | Member of the audit committee. |
| (2) | Member of the compensation committee. |
Paul E. Colombo founded Applied Epi in 1986 and has served as the Chairman of the board of directors of Applied Epi since its inception. He has held various positions with Applied Epi, most recently President until June 2000 and as Chief Executive Officer until July 2000.
David G. Reamer has been President of Applied Epi since June 2000, Chief Executive Officer since July 2000 and a Director since June 1999. From June 1999 until he re-joined Applied Epi in March 2000, Mr. Reamer served as Director of Business Development for ADC Telecommunications. From April 1994 to June 1999, he held a number of positions in direct and indirect sales, technical support, product development and marketing with Applied Epi, most recently as Director of Sales and Marketing.
Marlin A. Braun has been Vice President of Strategic Marketing and Business Development of Applied Epi since April 2000. From August 1983 to April 2000, Mr. Braun held a variety of positions including sales, marketing, service and business development at Physical Electronics, Inc., most recently as Senior Manager — Asia Pacific and North America.
Brett D. Heffes has been Chief Financial Officer and Treasurer of Applied Epi since July 2000. From January 1998 to July 2000, Mr. Heffes was with Department 56, Inc., most recently as Vice President Corporate Development and Treasurer. From May 1992 to December 1997, he held a number of positions with Wessels, Arnold & Henderson, LLC, an investment banking firm, most recently as Principal. Mr. Heffes is a director for the J. Jill Group, Inc.
Frank C. Kraemer has been Chief Operating Officer of Applied Epi since July 2000 and a Director since June 1999. From June 1995 to July 2000, Mr. Kraemer was Chief Financial Officer of Applied Epi.
William J. Cadogan has been a Director of Applied Epi since September 2000. Since 1991, Mr. Cadogan has been a director and the President and Chief Executive Officer of ADC Telecommunications, Inc., a Minnetonka, MN, based designer and manufacturer of products and
48
Noel P. Rahn has been a Director of Applied Epi since January 2000. Mr. Rahn is currently, and since July 1998 has been, the principal of The Rahn Group, a venture capital firm. From April 1974 until June 1998, he served as the Chief Executive Officer of Investment Advisors, Inc., an investment management firm.
Board of Directors
Our bylaws provide that our board of directors must consist of at least three but not more than twelve directors. Our board of directors currently consists of five members. Our board of directors is divided into three classes, with staggered three-year terms of office. At each annual meeting of shareholders, directors who are elected to succeed the class of directors whose terms expired at that meeting will be elected for three-year terms. Mr. Colombo will be up for reelection at the 2001 annual meeting of shareholders, Messrs. Cadogan and Kraemer will be up for reelection at the 2002 annual meeting of shareholders, and Messrs. Rahn and Reamer will be up for reelection at the 2003 annual meeting of shareholders. Vacancies may be filled by a majority of the directors then in office. Each director holds office until their term expires or until their successor is duly elected.
Committees
Our board of directors has an audit committee and a compensation committee. The audit committee consists of Messrs. Cadogan and Rahn. The audit committee reviews our internal accounting procedures, consults with and reviews the services provided by our independent accountants and makes recommendations to the board of directors regarding the selection of independent accountants. The compensation committee consists of Messrs. Cadogan and Rahn. The compensation committee reviews and recommends to the board of directors the salaries, incentive compensation and benefits of our officers and employees and administers our stock plans and employee benefit plans.
Compensation Committee Interlocks and Insider Participation
Our board of directors established the compensation committee in September 2000. None of its members has been our officer or employee and there are no interlocking relationships between our compensation committee or executive officers and the board of directors, compensation committee or the executive officers of any other company.
Compensation
In July 2000, our board of directors approved compensation guidelines for directors who are not our officers or employees. These directors will be paid $1,000 per board meeting attended in person, $500 per telephonic meeting and $500 per committee meeting held on a date on which no board meeting is held, in addition to reimbursement for expenses incurred in attending any board of directors or committee meeting. Directors who are also our officers or employees will not receive separate compensation for attending board of directors or committee meetings. Our non-employee directors also receive in connection with their appointment or election to our board of directors an option to purchase 120,000 shares of our common stock under our 2000 Stock Plan, of which 40,000 shares vests on each of the first three anniversaries of the date of grant. The exercise price of the option is the estimated fair market value of our common stock on the date of grant and the option may be exercised until 30 days after the individual ceases to be a director.
49
Executive Officers
Compensation
The following table and accompanying footnotes summarize the compensation paid to our Chief Executive Officer and the only other executive officer whose total salary and bonus exceeded $100,000 for the year ended December 31, 1999. These individuals are referred to as our “named executive officers.”
Summary Compensation Table
| Long-Term | |||||||||||||||||
| Compensation | |||||||||||||||||
| Awards | |||||||||||||||||
| Annual Compensation | |||||||||||||||||
| Shares | |||||||||||||||||
| Other | Underlying | ||||||||||||||||
| Name and Position | Salary | Bonus | Compensation | Options | |||||||||||||
| Paul E. Colombo(1) | $ | 106,824 | $ | 0 | $ | 0 | — | ||||||||||
| Chairman of the Board | |||||||||||||||||
| Frank C. Kraemer | $ | 148,615 | $ | 0 | $ | 0 | — | ||||||||||
| Chief Operating Officer and Secretary | |||||||||||||||||
| (1) | Mr. Colombo served as the Chief Executive Officer of Applied Epi until July 2000. |
Option Grants in Fiscal Year 1999
No options were granted to the named executive officers during fiscal year 1999.
Aggregate Option Exercises in Fiscal Year 1999 and Values at December 31, 1999
The named executive officers did not exercise any stock options in 1999 nor did they hold any stock options as of December 31, 1999.
Employment Agreements
We do not have employment agreements with any of our named executive officers.
Limitations on Directors’ and Officers’ Liability and Indemnification
Our amended and restated articles of incorporation eliminate the personal liability of our directors to us and our shareholders for monetary damages for breach of fiduciary duty, except liability associated with any of the following:
| • | any breach of their duty of loyalty to us or our shareholders; | |
| • | acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; | |
| • | unlawful payments of dividends or unlawful stock repurchases or redemptions; or | |
| • | any transaction from which the director derived an improper personal benefit. |
50
Stock Plans
1993 Stock Option Plan
Our 1993 Stock Option Plan was adopted by our board of directors and shareholders on June 25, 1993. This stock plan provides for the grant of incentive stock options and nonstatutory stock options to our key employees. As of September 25, 2000, we had 1,952,248 shares subject to outstanding options to purchase shares of common stock at a weighted average exercise price of $4.92 per share under our 1993 Stock Option Plan. No options granted under our 1993 Stock Option Plan have been exercised. In connection with the adoption of our 2000 Stock Plan described below, we decided we would not grant additional options under the 1993 Stock Option Plan. The 1993 Stock Option Plan remains in place to facilitate administration and exercise of the outstanding options.
Number of Shares of Common Stock Available under Our 1993 Stock Option Plan. There were 6,000,000 shares originally reserved for issuance of options under the 1993 Stock Option Plan.
Administration of Our 1993 Stock Option Plan. Our board of directors or a committee of our board of directors administers the 1993 Stock Option Plan. The committee must consist of two or more directors, none of whom is an employee or a former officer and none of whom receives compensation from us exceeding certain limits. The administrator has the power to determine the terms of the options granted, including the exercise price, the number of shares subject to each option, the exercisability of the options, and the form of consideration payable upon exercise. The 1993 Stock Option Plan is currently administered by our compensation committee.
Options. The administrator determines the exercise price and term of options granted under our 1993 Stock Option Plan. With respect to incentive stock options, however, the exercise price must at least be equal to the fair market value of our common stock on the date of grant and its term may not exceed ten years. The exercise price of an incentive stock option granted to a person who owns at least 10% of our stock must at least be equal to 110% of the fair market value of our common stock on the date of grant and its term may not exceed five years.
The aggregate fair market value of common stock subject to incentive stock options that are exercisable for the first time by the employee during any calendar year may not exceed $100,000. No individual may be granted an option to purchase more than 1,000,000 shares in any fiscal year.
After termination of employment, the option terminates. If termination is due to death or disability, the option will remain exercisable for three months following the termination. However, an option may never be exercised later than the expiration of its term.
Transferability of Options. Our 1993 Stock Option Plan does not allow for the transfer of options otherwise than by will or the laws of descent and distribution. During the employee’s lifetime, only the employee may exercise his or her option.
Adjustments upon Merger, Asset Sale or Similar Change of Control Event. Our 1993 Stock Option Plan provides that in the event of a merger, consolidation, acquisition, separation, reorganization, liquidation (other than bankruptcy), dissolution, the transfer of substantially all of the assets or 75% of our stock or like occurrence, all options become fully exercisable for a period specified by the plan administrator, which shall not exceed sixty days nor be less than seven days prior to such event.
Termination and Amendment. According to its terms, the 1993 Stock Option Plan has no expiration and incentive stock options may not be granted after June 25, 2003. However, as indicated before, we do not intend to grant any additional options under this plan. In addition, our board of directors has the authority to amend or terminate the 1993 Stock Option Plan provided that
51
2000 Stock Plan
Our 2000 Stock Plan provides for the grant of incentive stock options, nonstatutory stock options and restricted stock awards to our officers, other key employees, consultants and directors, including non-employee directors. Only our employees are eligible for incentive stock options and only officers and key employees are eligible for restricted stock awards. As of September 25, 2000, we had 242,000 shares subject to outstanding options to purchase shares of common stock at an exercise price of $12.50 per share under our 2000 Stock Plan. No options granted under our 2000 Stock Plan have been exercised and no restricted stock awards are currently outstanding.
Number of Shares of Common Stock Available under Our 2000 Stock Plan. There are 6,000,000 shares reserved for issuance of options under the 2000 Stock Plan.
Administration of Our 2000 Stock Plan. Our board of directors or a committee of our board of directors administers the 2000 Stock Plan. The committee must consist of two or more directors, none of whom is an employee or a former officer and none of whom receives compensation from us exceeding certain limits. The administrator has the power to determine the terms of the options or restricted stock awards granted, including the exercise price, the number of shares subject to each option or restricted stock award, the exercisability of the options, the lapsing of restrictions on restricted stock awards, and the form of consideration payable upon exercise. The 2000 Stock Plan is currently administered by our compensation committee.
Options. The administrator determines the exercise price and term of options granted under our 2000 Stock Plan. With respect to incentive stock options, however, the exercise price must at least be equal to the fair market value of our common stock on the date of grant and its term may not exceed ten years. The exercise price of an incentive stock option granted to a person who owns at least 10% of our stock must at least be equal to 110% of the fair market value of our common stock on the date of grant and its term may not exceed five years.
The aggregate fair market value of common stock subject to incentive stock options that are exercisable for the first time by the employee during any calendar year may not exceed $100,000. No individual may be granted an option to purchase more than 1,000,000 shares in any fiscal year.
After termination of employment or retirement, the option will remain exercisable for three months following such termination. If termination is due to death, the option will remain exercisable for twelve months following death. If termination is due to disability, the option will remain exercisable for nine months following the date of termination due to the disability. However, an option may never be exercised later than the expiration of its term.
Restricted Stock Awards. We may issue shares of restricted stock under our 2000 Stock Plan. The plan administrator determines the terms and conditions which cause restrictions to lapse with respect to specific shares.
Transferability of Options and Restricted Stock Awards. Our 2000 Stock Plan does not allow for the transfer of options otherwise than by will or the laws of descent and distribution, or pursuant to certain other limited exceptions, such as a qualified domestic relations order. During the optionee’s lifetime, only the optionee may exercise his or her option. Upon termination of employment for any reason during a time when the employee’s restricted stock award is still subject to restrictions, all shares still subject to those restrictions are forfeited.
52
Adjustments upon Merger, Asset Sale or Similar Change of Control Event. Our 2000 Stock Plan provides that in the event of a merger, consolidation, acquisition, separation, reorganization, liquidation (other than bankruptcy), dissolution, the transfer of substantially all of the assets or 75% of our stock or like occurrence, all options become fully exercisable for a period specified by the plan administrator, which shall not exceed sixty days nor be less than seven days prior to such event. Any of these events will also cause all restrictions to lapse for any restricted stock award on the tenth business day prior to such event.
Termination and Amendment. The 2000 Stock Plan has no expiration. Incentive stock options may not be granted after July 31, 2010. In addition, our board of directors has the authority to amend, alter or terminate the 2000 Stock Plan, provided that we will need the participant’s consent to make a change in the terms of an option or a restricted stock award which would impair the rights of that participant. In addition, shareholder approval is required to increase the number of shares of common stock reserved for the issuance of options under the plan.
Employee Performance Stock Ownership Plan
Our Employee Performance Stock Ownership Plan was adopted in June 1993 and will terminate with this offering. This plan is a phantom stock plan pursuant to which we have made discretionary awards of performance units to our full-time employees who had at least one year of service with us. Performance units granted to a participant vest upon grant and are payable on the earlier of March 15th of the third calendar year after the grant, the participant’s death, disability or retirement and upon a change in control resulting from a sale, merger or issuance of shares. The value of each performance unit was determined at the end of each year based on our cumulative pre-tax profits, as defined, for the three-year period then ended. Vested units distributed in a given year would have been paid in cash at the value per unit determined on the last calendar year end. Most participants have elected to defer receipt of the payments under this Plan.
In connection with this offering, we will distribute shares of our common stock and cash as payment for all outstanding performance units. The holder of each performance unit will receive one-half share of common stock and an equivalent value of cash for each performance unit held and the cash portion will be withheld for tax payments attributable to the distribution. Transfer of the shares will be restricted for periods ranging from six months to three years, depending on when the performance unit was initially granted. We expect to issue approximately 621,482 shares and distribute approximately $ in payment for the performance units currently outstanding upon completion of this offering.
401(k) Plan
We adopted a 401(k) Plan effective April 1995 covering our employees. Employees become eligible to participate in the 401(k) Plan after their first month of employment. The 401(k) Plan is intended to qualify under Section 401(k) of the United States Internal Revenue Code, so that contributions to the 401(k) Plan by employees or by us and the investment earnings thereon are not taxable to the employees until withdrawn. If our 401(k) Plan qualifies under Section 401(k) of the United States Internal Revenue Code, our contributions will be deductible by us when made. Our employees may elect to reduce their current compensation by up to the statutorily prescribed annual limit of $10,500 in 2000 and to have those funds contributed to the 401(k) Plan. The 401(k) Plan permits us, but does not require us, to make additional matching contributions on behalf of all participants. To date, we have not made any contributions to the 401(k) Plan.
53
Distributions to Shareholders
Since 1997, we have been treated for federal and state income tax purposes as an S corporation under Subchapter S of the Internal Revenue Code of 1986 and comparable state tax laws. As a result, our earnings are taxed and will be taxed until the termination of our S corporation status, with certain exceptions, directly to the existing shareholders rather than to us, leaving the shareholders responsible for paying income taxes on these earnings. We have historically paid distributions to our shareholders to enable them to pay their income tax liabilities as a result of our status as an S corporation and, from time to time, to distribute previously undistributed S corporation earnings and profits. During 1998, we made S corporation distributions to our shareholders of approximately $4.0 million. We did not make any distributions in 1997 or 1999. We intend to terminate our S corporation status prior to the closing of this offering. We intend to distribute approximately $6.0 million for the net proceeds of this offering to our S corporation shareholders, representing S corporation income that had been taxed to our shareholders and not previously distributed to them, plus an additional amount equal to our estimated taxable income earned during 2000 prior to the conversion to a C corporation.
Spectracom Transactions
In July 1997, Spectracom, Inc. was formed for purposes of developing high power laser pump applications for the telecommunications industry. Spectracom is majority owned by our Chairman, Mr. Colombo, and its board of directors and executive officers include Messrs. Colombo, Reamer and Kraemer. During 1998, we were reimbursed by Spectracom for certain costs incurred on behalf of Spectracom in the amount of $179,000.
During 1998 and 1999, Spectracom purchased approximately $1.8 million of MBE products from us for use in its development operations. The prices charged to Spectracom were substantially the same as those charged to our unrelated customers.
During 1998 and until June 1999, we advanced $9.9 million to Spectracom. The advances to Spectracom were at a favorable interest rate to Spectracom, for which Spectracom compensated Applied Epi by issuing 3,070,880 shares of Spectracom’s common stock to us. The Spectracom stock was distributed to our shareholders, of which Mr. Colombo received 2,679,165 shares, Mr. Reamer received 148,212 shares and Mr. Kraemer received 149,938 shares. In June 1999, Spectracom’s assets were sold to ADC Telecommunications, Inc., and all amounts due to us were repaid.
Purchase of Subsidiary Stock
In order to make our affiliates, Applied Epi International, Inc. and Applied Epi Europe, Inc., our wholly-owned subsidiaries, on September 25, 2000, we purchased all of the issued and outstanding stock of each of those companies from their sole shareholder, Mr. Colombo. Specifically, we purchased from Mr. Colombo 50,000 shares of Applied Epi Europe’s common stock and 250 shares of Applied Epi International’s common stock. As payment for these shares, we issued 97,604 shares of our common stock to Mr. Colombo valued at $12.50 per share. For accounting purposes, this transaction was treated on an “as if pooling” basis (see Note 1 of Notes to Consolidated Financial Statements).
54
Loans from Officers
In 1997 and 1998, we borrowed approximately $2.1 million from our Chairman, Mr. Colombo. The interest charged for each of these loans was at the prime rate plus 0.5%. The principal and interest on these notes was repaid in 1999. In 1999, Mr. Colombo provided an additional loan to us of $500,000, which had an interest rate of prime plus 0.5%. Principal and interest on this loan was repaid in April 2000.
Loans to Affiliates
As of December 31, 1999, we had advanced approximately $589,932 to NuVision, LLC for purposes of product development. The entire balance was repaid by NuVision in April 2000. NuVision is majority-owned by our Chairman, Mr. Colombo.
Officer’s Employment Agreement
In July 2000, we entered into an employment agreement with Mr. Heffes, our Chief Financial Officer and Treasurer, which provides for an annual salary of $200,000, prohibits him from competing against us for 18 months from the date of his termination and contains certain confidentiality restrictions. The agreement also provides that we will pay Mr. Heffes his salary and bonus for one year if he is terminated without cause, relocated or experiences a diminution of title or responsibilities. We also granted Mr. Heffes a ten-year option to purchase 240,000 shares at an exercise price of $10.00 per share. His option vests equally over a three-year period commencing one year after the grant date, unless there is a change in control in which case his entire option vests immediately.
Loan Guarantees
In 1997 and 1998, we and our subsidiaries entered into a credit facility with U.S. Bank National Association allowing us to borrow up to approximately $8.6 million from U.S. Bank National Association. Our Chairman, Mr. Colombo, provided the bank with a guaranty for the full amount of these loans, which were paid in full in June 1999.
In December 1999, we entered into a $4.5 million loan agreement with Jackson National Life Insurance Company. Applied Epi International, Inc., Applied Epi Europe, Inc. and Mr. Colombo guaranteed this loan.
On June 5, 2000, we and our subsidiaries entered into a credit agreement with U.S. Bank National Association providing us with a revolving loan of up to $2.5 million. Mr. Colombo has provided U.S. Bank with a guaranty for this loan. In addition, on July 31, 2000, we received a $1.7 million loan from U.S. Bank National Association. Mr. Colombo, Applied Epi Europe, Inc. and Applied Epi International, Inc. have each guaranteed this loan.
55
The following table sets forth information known to us with respect to the beneficial ownership of our common stock as of September 25, 2000, and as adjusted to reflect the sale of common stock offered hereby by the following:
| • | each shareholder known by us to own beneficially more than 5% of our common stock; | |
| • | each of our executive officers named in the compensation table above; | |
| • | each of our directors; and | |
| • | all directors and executive officers as a group. |
As of September 25, 2000, there were 21,602,980 shares of our common stock outstanding. Except as otherwise indicated, we believe that the beneficial owners of the common stock listed below, on the information furnished by such owners, have sole voting power and investment power with respect to such shares. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission. In computing the number of shares beneficially owned by a person and the percent ownership of that person, shares of common stock subject to options or warrants held by that person that are currently exercisable or will become exercisable within 60 days after September 25, 2000 are deemed outstanding, while such shares are not deemed outstanding for purposes of computing percent ownership of any other person. The address for those individuals for which an address is not otherwise indicated in the table below is Applied Epi, Inc., 4900 Constellation Drive, St. Paul, MN 55127.
| Percentage of | |||||||||||||
| Shares Outstanding | |||||||||||||
| Beneficially Owned | |||||||||||||
| Shares Beneficially | |||||||||||||
| Owned Prior to | Prior to | After | |||||||||||
| Name of beneficial owner | Offering | Offering | Offering | ||||||||||
| Paul E. Colombo | 17,450,084 | 80.8 | % | % | |||||||||
| Frank C. Kraemer(1) | 1,515,020 | 6.9 | % | ||||||||||
| David G. Reamer(1) | 2,014,468 | 9.0 | % | ||||||||||
| Noel P. Rahn | 689,280 | 3.2 | % | ||||||||||
|
3355 U.S. Bank Place 601 Second Avenue S. Minneapolis, MN 55402 |
|||||||||||||
| William J. Cadogan | — | — | — | ||||||||||
|
12501 Whitewater Drive Minnetonka, MN 55343 |
|||||||||||||
| Directors and executive officers as a group (7 persons, including those named above) | 21,722,612 | 96.5 | % | % | |||||||||
| (1) | Includes the following shares issuable upon exercise of options that are exercisable within 60 days as follows: Mr. Kraemer, 200,000 and Mr. Reamer, 699,448. |
56
We are authorized to issue 125,000,000 shares, consisting of 100,000,000 shares of common stock and 25,000,000 shares of preferred stock. The following description of our capital stock is only a summary. You should refer to our amended and restated articles of incorporation and bylaws as in effect upon the closing of this offering, which are included as exhibits to the registration statement of which this prospectus forms a part, and the provisions of applicable Minnesota law.
Common Stock
As of September 25, 2000, we had 21,602,980 shares of common stock outstanding held by 21 shareholders of record. Based upon the number of shares outstanding as of September 25, 2000, and giving effect to the issuance of the shares being offered by us (assuming no exercise of the underwriters’ over-allotment option and no exercise of outstanding options), we will have shares of common stock outstanding upon closing of the offering.
As of September 25, 2000, there were 1,952,248 shares issuable upon exercise of outstanding options under our 1993 Stock Option Plan, and 242,000 shares issuable upon exercise of outstanding options under our 2000 Stock Plan. See “Management — Stock Plans” for a description of our stock plans.
The holders of our common stock are entitled to one vote per share held of record on all matters submitted to a vote of the shareholders. Our amended and restated articles of incorporation do not provide for cumulative voting in the election of directors. Subject to preferences that may be applicable to any outstanding preferred stock, the holders of common stock are entitled to receive ratably dividends, if any, as may be declared from time to time by our board of directors out of funds legally available for that purpose. In the event of our liquidation, dissolution or winding up, holders of our common stock are entitled to share ratably in all assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock, if any, then outstanding. Holders of our common stock have no preemptive or other subscription or conversion rights. There are no redemption or sinking fund provisions applicable to our common stock. All outstanding shares of common stock are fully paid and non-assessable, and the shares of common stock to be issued upon the completion of this offering will be fully paid and non-assessable.
Preferred Stock
Our board of directors is authorized, without action by the shareholders, to issue 25,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges may include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting any series or the designation of any series, all or any of which may be greater than the rights of the common stock.
The issuance of preferred stock could adversely affect the voting power of holders of common stock and the likelihood that the holders of common stock will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying or preventing a change in our control without further action by the shareholders. We have no present plans to issue any shares of preferred stock.
57
Minnesota Anti-Takeover Law and Certain Charter and Bylaw Provisions
Certain provisions of Minnesota law and our amended and restated articles of incorporation and bylaws could make it more difficult for third parties to acquire control of Applied Epi by means of a tender offer, a proxy contest or otherwise and could also make the removal of incumbent officers and directors more difficult. These provisions, summarized below, are expected to discourage certain types of coercive takeover practices and inadequate takeover bids, and are intended to provide management with flexibility, enhance the likelihood of continuity and stability in the composition of our board of directors and the policies of our board and to discourage an unsolicited takeover of our company if our board of directors determines that the takeover is not in the best interests of our company and our shareholders. However, these provisions could have the effect of discouraging attempts to acquire us, which could deprive our shareholders of opportunities to sell their shares of common stock at prices higher than prevailing market prices. We believe that the benefits of increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweighs the disadvantages of discouraging such proposals because, among other things, negotiation of such proposals could result in an improvement of their terms.
Undesignated Preferred Stock
The existence of authorized but unissued preferred stock makes it possible for the board of directors to issue preferred stock with voting or other rights or preferences that could make it more difficult for third parties to acquire control of Applied Epi.
Anti-Takeover Provisions of Minnesota Statutes
Section 302A.671 of Minnesota Statutes applies, with certain exceptions, to any acquisition of our voting stock from a person other than us, and other than in connection with certain mergers and exchanges to which we are a party, resulting in the beneficial ownership in excess of 20%, 33 1/3% or 50% of our voting power. The shares acquired in excess of any of these percentage thresholds will have no voting rights unless the share acquisition is approved by a majority of our disinterested shareholders. We are required to hold a special shareholders’ meeting to vote on the acquisition after the acquiror delivers to us an information statement describing, among other things, the acquiror and any plans of the acquiror to liquidate or dissolve us, and copies of definitive financing agreements for any financing of the acquisition not to be provided by funds of the acquiror. If the acquiror does not submit an information statement to us within ten days after acquiring shares representing a new threshold percentage of voting control, or if our disinterested shareholders vote not to approve the acquisition, we may redeem the shares at their fair market value.
Section 302A.673 of Minnesota Statutes generally prohibits any business combination by us, or any subsidiary of us, with any shareholder that purchases 10% or more of our voting shares in the four years following the acquisition, unless a majority of our disinterested directors have approved either the business combination or the acquisition of shares that made the shareholder a 10% holder, in each case before the business combination or the 10% acquisition. Section 302A.673 defines a business combination broadly to include, among other things, mergers, sales of substantial assets, loans, substantial issuances of stock and various other transactions involving us and the interested shareholder or its affiliates.
Section 302A.675 of the Minnesota Statutes generally prohibits an offeror from acquiring our shares within two years following the offeror’s last purchase of our shares pursuant to a takeover offer, unless our shareholders are entitled to sell their shares to the offeror upon substantially equivalent terms as those provided in the earlier takeover offer.
58
Charter Provisions
Our board of directors is divided into three classes, serving staggered three-year terms. As a result of this division, generally at least two shareholders’ meetings will be required for shareholders to effect a change in control of the board of directors. Also, our shareholders may remove a director only by a 70% vote. The board of directors may remove a director by a majority vote, but only if the removed director had not yet been elected by the shareholders. In addition, our amended and restated bylaws contain certain procedures that must be followed before a shareholder may bring matters for consideration at a shareholders’ meeting or nominate a director for election, including giving us advance notice within specified time periods and providing us with certain other information regarding the matter to be acted upon.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Wells Fargo Bank Minnesota, N.A.
Nasdaq Stock Market National Market Listing
We have applied for quotation of our common stock on the Nasdaq Stock Market’s National Market under the symbol “APEP.”
59
Prior to this offering, there has been no public market for our common stock. Future sales of substantial amounts of our common stock in the public market following this offering, or the possibility of such sales occurring, could adversely affect the prevailing market price of our common stock and our ability to raise equity capital in the future.
Upon completion of this offering, we will have shares of common stock outstanding, based on the number of shares outstanding as of September 25, 2000 and assuming no exercise of the underwriters’ over-allotment option and no exercise of outstanding options. Of these shares, the shares to be sold in this offering will be freely tradable in the public market without restriction under the Securities Act, unless such shares are held by “affiliates” of Applied Epi, as that term is defined in Rule 144 under the Securities Act.
The remaining shares outstanding held by existing shareholders are “restricted securities” as that term is defined under Rule 144. We issued and sold the restricted shares in private transactions in reliance on exemptions from registration under the Securities Act. Restricted shares may be sold in the public market only if they are registered or if they qualify for an exemption from registration under Rule 144 or Rule 701 under the Securities Act, as summarized below.
Lock-up Agreements
In connection with this offering, our executive officers, directors, shareholders and option holders have agreed with the underwriters that, subject to exceptions, they will not sell or dispose of any of their shares for 180 days after the date of this prospectus without the written consent of Donaldson, Lufkin and Jenrette Securities Corporation and Merrill Lynch. Donaldson, Lufkin & Jenrette Securities Corporation and Merrill Lynch may at any time without notice, release all or any portion of the shares subject to such restrictions. The shares of common stock outstanding upon closing of this offering will be available for sale in the public market as follows:
| Approximate Number | ||||||
| Days after the Date | of Shares Eligible | |||||
| of this Prospectus | for Future Sale | Description | ||||
| Upon effectiveness | Freely tradable shares sold in this offering. | |||||
| 180 days | Lock-up released; shares saleable under Rule 144, 144(k) or 701 | |||||
| Over 180 days | Restricted securities held for less than one year or subject to other transfer restrictions | |||||
Rule 144
In general, under Rule 144 as currently in effect, beginning 90 days after the date of this prospectus, a person who has beneficially owned shares of our common stock for at least one year from the later of the date those shares were acquired from us or from an affiliate of ours would be entitled to sell within any three-month period, a number of shares that does not exceed the greater of:
| • | 1% of the number of shares of common stock then outstanding, which will equal approximately shares immediately after this offering; or | |
| • | the average weekly trading volume of our common stock on the Nasdaq National Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale of any shares of common stock. |
60
The sales of any shares of our common stock under Rule 144 are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us.
Rule 144(k)
Under Rule 144(k), a person who is not one of our affiliates at any time during the three months preceding a sale, and who has beneficially owned the shares proposed to be sold for at least two years from the later of the date such shares of common stock were acquired from us or from an affiliate of ours, including the holding period of any prior owner other than an affiliate, is entitled to sell those shares without complying with the manner of sale, public information, volume limitation or notice provisions of Rule 144. Therefore, unless otherwise restricted pursuant to the lock-up agreements or otherwise, those shares may be sold immediately upon the completion of this offering.
Rule 701
In general, under Rule 701 of the Securities Act as currently in effect, each of our employees, consultants or advisors who purchased shares from us in connection with a compensatory stock plan or other written agreement is eligible to resell those shares 90 days after the effective date of this offering in reliance on Rule 144, but without compliance with some of the restrictions, including the holding period, contained in Rule 144. Shares issued under our Employee Performance Stock Ownership Plan may be sold in reliance on Rule 701, subject to restrictions on transfer ranging from six months to three years.
No precise prediction can be made as to the effect, if any, that market sales of shares or the availability of shares for sale pursuant to Rules 144, 144(k) or 701 will have on the market price of our common stock prevailing from time to time. We are unable to estimate the number of our shares that may be sold in the public market pursuant to Rules 144, 144(k) or Rule 701 because this will depend on the market price of our common stock, the personal circumstances of the sellers and other factors. Nevertheless, sales of significant amounts of our common stock in the public market could adversely affect the market price of our common stock.
Stock Plans
As soon as practicable following the completion of this offering, we intend to file registration statements on Form S-8 under the Securities Act to register shares of our common stock reserved for issuance under our 1993 Stock Option Plan and 2000 Stock Plan. These registrations will permit the resale of these shares by nonaffiliates in the public market without restriction under the Securities Act, upon completion of the lock-up period described above. Shares registered under the Form S-8 registration statement held by affiliates will be subject to Rule 144 volume limitations as well as the lock-up agreements described above.
61
We intend to offer the shares of common stock described in this prospectus through a number of underwriters. Donaldson, Lufkin & Jenrette Securities Corporation, Merrill Lynch, Pierce, Fenner & Smith Incorporated and Wit SoundView Corporation are acting as representatives of the underwriters named below. Subject to the terms and conditions described in a purchase agreement among us and the underwriters, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase from us, the number of shares listed next to its name below.
| Number of | |||||
| Underwriter | Shares | ||||
| Donaldson, Lufkin & Jenrette Securities Corporation | |||||
| Merrill Lynch, Pierce, Fenner & Smith Incorporated | |||||
| Wit SoundView Corporation | |||||
| Total | |||||
The underwriters have agreed to purchase all of the shares sold under the purchase agreement if any of these shares are purchased. If an underwriter defaults, the purchase agreement provides that the purchase commitments of the nondefaulting underwriters may be increased or the purchase agreement may be terminated.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make in respect of those liabilities.
The underwriters are offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the shares, and other conditions contained in the purchase agreement, such as the receipt by the underwriters of officers certificates and legal opinions. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Commissions and Discounts
The representatives have advised us that the underwriters propose initially to offer the shares to the public at the initial public offering price on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per share. The underwriters may allow, and the dealers may reallow, a discount not in excess of $ per share to other dealers. After the initial public offering, the public offering price, concession and discount may be changed.
The following table shows the public offering price, underwriting discount and proceeds before expenses to Applied Epi. The information assumes either no exercise or full exercise by the underwriters of their over-allotment options.
| Per Share | Without Option | With Option | |||||||||||
| Public offering price | $ | $ | $ | ||||||||||
| Underwriting discount | $ | $ | $ | ||||||||||
| Proceeds, before expenses, to | |||||||||||||
| Applied Epi | $ | $ | $ | ||||||||||
The expenses of the offering, not including the underwriting discount, are estimated at $ and are payable by Applied Epi.
62
Over-allotment Option
We have granted options to the underwriters to purchase up to additional shares at the public offering price less the underwriting discount. The underwriters may exercise these options for 30 days from the date of this prospectus solely to cover any over-allotments. If the underwriters exercise these options, each will be obligated, subject to conditions contained in the purchase agreement, to purchase a number of additional shares proportionate to that underwriter’s initial amount reflected in the above table.
Reserved Shares
At our request, the underwriters have reserved for sale, at the initial public offering price, up to shares, or 5.0% of the shares offered by this prospectus for sale to some of our directors, officers, employees, business associates and related persons. If these persons purchase reserved shares, this will reduce the number of shares available for sale to the general public. Any reserved shares that are not orally confirmed for purchase within one day of the pricing of the offering will be offered by the underwriters to the general public on the same terms as the other shares offered by this prospectus.
No Sales of Similar Securities
We and our executive officers, directors, shareholders and option holders have agreed, with exceptions, not to sell or transfer any shares of common stock for 180 days after the date of this prospectus without first obtaining the written consent of Donaldson, Lufkin & Jenrette Securities Corporation and Merrill Lynch. Specifically, we and these other individuals have agreed not to directly or indirectly:
| • | offer, pledge, sell or contract to sell any shares of common stock, | |
| • | sell any option or contract to purchase any shares of common stock, | |
| • | purchase any option or contract to sell any shares of common stock, | |
| • | grant any option, right or warrant for the sale of any shares of common stock, | |
| • | lend, pledge or otherwise dispose of or transfer any shares of common stock, | |
| • | request or demand that we file a registration statement related to the shares of common stock, or | |
| • | enter into any swap or other agreement that transfers, in whole or in part, the economic consequence of ownership of any shares of common stock whether any such swap or transaction is to be settled by delivery of shares or other securities, in cash or otherwise. |
This lockup provision applies to shares of common stock and to securities convertible into or exchangeable or exercisable for or repayable with shares of common stock. It also applies to shares of common stock owned now or acquired later by the person executing the agreement or for which the person executing the agreement later acquires the power of disposition.
Quotation on the Nasdaq National Market
We expect the shares to be approved for quotation on the Nasdaq National Market under the symbol “APEP.” Before this offering, there has been no public market for our common stock. The initial public offering price will be determined through negotiations among us, the representatives and
63
| • | the valuation multiples of publicly traded companies that the representatives and the lead managers believe to be comparable to us, | |
| • | our financial information, | |
| • | the history of, and the prospects for, our company and the industry in which we compete, | |
| • | an assessment of our management, its past and present operations, and the prospects for, and timing of, our future revenues, | |
| • | the present state of our development, | |
| • | the prospects for our future earnings, and | |
| • | the above factors in relation to market values and various valuation measures of other companies engaged in activities similar to ours. |
An active trading market for the shares may not develop. It is also possible that after the offering the shares will not trade in the public market at or above the initial public offering price.
The underwriters do not expect to sell more than 5% of the shares in the aggregate to accounts over which they exercise discretionary authority.
Price Stabilization, Short Positions and Penalty Bids
Until the distribution of the shares is completed, SEC rules may limit underwriters from bidding for and purchasing our common stock. However, the representatives may engage in transactions that stabilize the price of the shares of common stock, such as bids or purchases to peg, fix or maintain that price.
If the underwriters create a short position in the shares of common stock in connection with the offering, i.e., if they sell more shares than are listed on the cover page of this prospectus, the representatives may reduce that short position by purchasing shares in the open market. The representatives may also elect to reduce any short position by exercising all or part of the over-allotment option described above. Purchases of the shares of common stock to stabilize its price or to reduce a short position may cause the price of the shares of common stock to be higher than it might be in the absence of such purchases.
The representatives may also impose a penalty bid on underwriters. This means that if the representatives purchase shares in the open market to reduce the underwriters’ short position or to stabilize the price of such shares, they may reclaim the amount of the selling concession from the underwriters who sold those shares. The imposition of a penalty bid may also affect the price of the shares in that it discourages resales of those shares.
Neither we nor any of the underwriters makes any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of the shares of common stock. In addition, neither we nor any of the underwriters makes any representation that the representatives or the lead managers will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
64
Electronic Prospectus
A prospectus in electronic format will be made available on websites maintained by one or more of the underwriters participating in this offering, including Donaldson, Lufkin & Jenrette Securities Corporation’s affiliate, DLJdirect Inc. and Wit SoundView Corporation’s affiliate, Wit Capital Corporation. In addition, other dealers purchasing shares from Wit SoundView in this offering have agreed to make a prospectus in electronic format available on websites maintained by each of these dealers.
Other Relationships
From time to time, the underwriters or their affiliates may provide investment banking and commercial banking services to Applied Epi.
65
The validity of the shares of common stock offered by this prospectus will be passed upon for us by Robins, Kaplan, Miller & Ciresi L.L.P., Minneapolis, Minnesota. The underwriters are being represented by Latham & Watkins, Chicago, Illinois. As of the date of this prospectus, members of and persons associated with Robins, Kaplan, Miller & Ciresi L.L.P. beneficially own an aggregate of 4,000 shares of our common stock.
The consolidated financial statements as of December 31, 1998 and 1999, and for each of the three years in the period ended December 31, 1999, included in the prospectus have been audited by Deloitte & Touche LLP, independent auditors, as stated in their report appearing herein, and have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
This prospectus is part of a registration statement on Form S-1 that we filed with the SEC. This prospectus does not contain all of the information contained in the registration statement and all of its exhibits and schedules. For further information about us, please see the complete registration statement. Summaries of agreements or other documents referred to in this prospectus are not necessarily complete. Please refer to the exhibits to the registration statement for complete copies of these documents.
You may read and copy our registration statement and all of its exhibits and schedules at the following SEC public reference rooms:
| 450 Fifth Street, N.W. | Seven World Trade Center | Citicorp Center | ||
| Judiciary Plaza | Suite 1300 | 500 West Madison Street | ||
| Room 1024 | New York, NY 10048 | Suite 1400 | ||
| Washington, D.C. 20549 | Chicago, IL 60661 |
You may obtain information on the operation of the SEC public reference room in Washington, D.C. by calling the SEC at 1-800-SEC-0330. The registration statement is also available from the SEC’s website at http://www.sec.gov, which contains reports, proxy and information statements and other information regarding issuers that file electronically.
We intend to furnish our shareholders with annual reports containing consolidated financial statements audited by an independent public accounting firm and make available to our shareholders quarterly reports for the first three quarters of each fiscal year containing interim unaudited consolidated financial information.
66
APPLIED EPI, INC.
| Independent Auditors’ Report | F-2 | |||
| Consolidated Balance Sheets | F-3 | |||
| Consolidated Statements of Operations | F-4 | |||
| Consolidated Statements of Stockholders’ Equity (Deficit) | F-5 | |||
| Consolidated Statements of Cash Flows | F-6 | |||
| Notes to Consolidated Financial Statements | F-7 |
F-1
The accompanying consolidated financial statements give effect to the completed tax-free transaction whereby Applied Epi International, Inc. and Applied Epi Europe, Inc. became wholly owned subsidiaries of the Company and the completion of the four-for-one split of the Company’s outstanding common stock which will take place immediately prior to the effectiveness of the Company’s planned initial public offering. The following report is in the form which will be furnished by Deloitte & Touche LLP upon completion of the stock split of the Company’s outstanding common stock described in Note 1 to the consolidated financial statements and, assuming that from September 8, 2000 to the date of such completion, no other material events have occurred that would affect the accompanying consolidated financial statements or required disclosure therein.
“To the Board of Directors and Stockholders of
We have audited the accompanying consolidated balance sheets of Applied Epi, Inc. and Subsidiaries (the Company) as of December 31, 1998 and 1999, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 1999. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the consolidated financial position of Applied Epi, Inc. and Subsidiaries as of December 31, 1998 and 1999 and the consolidated results of their operations and their cash flows for each of the three years in the period ended December 31, 1999, in conformity with accounting principles generally accepted in the United States of America.
Minneapolis, Minnesota
/s/ DELOITTE & TOUCHE LLP
September 28, 2000
F-2
Applied Epi, Inc. and Subsidiaries
| June 30, 2000 | ||||||||||||||||||
| December 31, | ||||||||||||||||||
| Pro Forma | ||||||||||||||||||
| 1998 | 1999 | Actual | (Note 3) | |||||||||||||||
| (unaudited) | ||||||||||||||||||
| ASSETS | ||||||||||||||||||
| CURRENT ASSETS: | ||||||||||||||||||
| Cash and cash equivalents | $ | 1,798,915 | $ | 1,478,464 | $ | 1,885,499 | $ | 1,885,499 | ||||||||||
| Accounts receivable (less allowance for doubtful accounts of $60,000 at December 31, 1998 and 1999) | 2,505,600 | 2,939,386 | 2,807,107 | 2,807,107 | ||||||||||||||
| Due from related party | 589,932 | 589,932 | — | — | ||||||||||||||
| Inventories | 2,136,888 | 2,980,827 | 6,162,224 | 6,162,224 | ||||||||||||||
| Deferred income taxes | — | — | — | 439,000 | ||||||||||||||
| Prepaid expenses and advances | 234,475 | 105,123 | 134,853 | 134,853 | ||||||||||||||
| Total current assets | 7,265,810 | 8,093,732 | 10,989,683 | 11,428,683 | ||||||||||||||
| PROPERTY AND EQUIPMENT: | ||||||||||||||||||
| Land | — | 1,825,000 | 4,108,400 | 4,108,400 | ||||||||||||||
| Building | — | 4,280,040 | 4,280,040 | 4,280,040 | ||||||||||||||
| Equipment, furniture, and fixtures | 741,136 | 1,190,558 | 1,772,680 | 1,772,680 | ||||||||||||||
| Total | 741,136 | 7,295,598 | 10,161,120 | 10,161,120 | ||||||||||||||
| Less accumulated depreciation | 507,744 | 662,495 | 812,593 | 812,593 | ||||||||||||||
| Net property and equipment | 233,392 | 6,633,103 | 9,348,527 | 9,348,527 | ||||||||||||||
| TAX INCREMENT FINANCING RECEIVABLE | — | 719,960 | 719,960 | 719,960 | ||||||||||||||
| GOODWILL, net of accumulated amortization | 846,030 | 715,871 | 650,792 | 650,792 | ||||||||||||||
| DEFERRED INCOME TAXES | — | — | — | 539,000 | ||||||||||||||
| OTHER ASSETS, net of accumulated amortization | 25,953 | 127,508 | 122,886 | 122,886 | ||||||||||||||
| $ | 8,371,185 | $ | 16,290,174 | $ | 21,831,848 | $ | 22,809,848 | |||||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||||||||
| CURRENT LIABILITIES: | ||||||||||||||||||
| Current portion of long-term debt | $ | 7,813,801 | $ | 325,914 | $ | 2,282,391 | $ | 2,282,391 | ||||||||||
| Notes payable — related parties | 2,135,000 | 500,000 | — | — | ||||||||||||||
| Accounts payable | 319,854 | 494,396 | 1,191,117 | 1,191,117 | ||||||||||||||
| Deferred revenue | 863,574 | 945,251 | 2,181,036 | 2,181,036 | ||||||||||||||
| Accrued compensation | 187,400 | 311,726 | 322,956 | 322,956 | ||||||||||||||
| Current portion of employee performance plan | 495,124 | 623,252 | 590,310 | 590,310 | ||||||||||||||
| Accrued warranty expenses | 148,996 | 146,881 | 182,455 | 182,455 | ||||||||||||||
| Other accrued expenses | 111,793 | 31,700 | 176,118 | 176,118 | ||||||||||||||
| Total current liabilities | 12,075,542 | 3,379,120 | 6,926,383 | 6,926,383 | ||||||||||||||
| EMPLOYEE PERFORMANCE PLAN, less current portion | 402,261 | 638,360 | 798,654 | 798,654 | ||||||||||||||
| LONG-TERM DEBT, less current portion | — | 5,018,296 | 5,107,486 | 5,107,486 | ||||||||||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||||||||||||
| STOCKHOLDERS’ EQUITY: | ||||||||||||||||||
| Preferred stock, $.01 par value — 25,000,000 shares authorized, none issued and outstanding | — | — | — | — | ||||||||||||||
| Common stock, $.01 par value — 100,000,000 shares authorized, 21,602,980 shares issued and outstanding | 216,030 | 216,030 | 216,030 | 216,030 | ||||||||||||||
| Additional paid-in capital | 647,980 | 647,980 | 18,073,561 | 18,984,763 | ||||||||||||||
| Deferred stock-based compensation | — | — | (10,201,468 | ) | (10,201,468 | ) | ||||||||||||
| Note receivable from related party | (6,269,168 | ) | — | — | — | |||||||||||||
| Retained earnings | 1,298,540 | 6,390,388 | 911,202 | 978,000 | ||||||||||||||
| Total stockholders’ equity (deficit) | (4,106,618 | ) | 7,254,398 | 8,999,325 | 9,977,325 | |||||||||||||
| $ | 8,371,185 | $ | 16,290,174 | $ | 21,831,848 | $ | 22,809,848 | |||||||||||
See notes to consolidated financial statements.
F-3
Applied Epi, Inc. and Subsidiaries
| Six Months Ended | ||||||||||||||||||||||
| Year Ended December 31, | June 30, | |||||||||||||||||||||
| 1997 | 1998 | 1999 | 1999 | 2000 | ||||||||||||||||||
| (unaudited) | ||||||||||||||||||||||
| SALES | $ | 11,853,572 | $ | 15,532,937 | $ | 15,388,941 | $ | 6,849,385 | $ | 10,179,770 | ||||||||||||
| COST OF SALES (exclusive of $84,029 for the six months ended June 30, 2000 reported below as stock-based compensation) | 6,179,661 | 8,685,909 | 8,579,349 | 3,885,017 | 5,237,004 | |||||||||||||||||
| GROSS PROFIT | 5,673,911 | 6,847,028 | 6,809,592 | 2,964,368 | 4,942,766 | |||||||||||||||||
| OPERATING EXPENSES: | ||||||||||||||||||||||
| Selling expenses (exclusive of $205,307 for the six months ended June 30, 2000 reported below as stock-based compensation) | 1,497,782 | 1,617,483 | 1,828,934 | 759,719 | 1,053,596 | |||||||||||||||||
| General and administrative expenses (exclusive of $6,824,340 for the six months ended June 30, 2000 reported below as stock-based compensation) | 1,146,341 | 1,107,966 | 1,042,757 | 531,561 | 1,086,057 | |||||||||||||||||
| Research and development expenses (exclusive of $110,437 for the six months ended June 30, 2000 reported below as stock-based compensation) | 1,458,036 | 717,483 | 471,561 | 235,126 | 747,438 | |||||||||||||||||
| Stock-based compensation | — | — | — | — | 7,224,113 | |||||||||||||||||
| Total operating expenses | 4,102,159 | 3,442,932 | 3,343,252 | 1,526,406 | 10,111,204 | |||||||||||||||||
| INCOME (LOSS) FROM OPERATIONS | 1,571,752 | 3,404,096 | 3,466,340 | 1,437,962 | (5,168,438 | ) | ||||||||||||||||
| INTEREST AND OTHER INCOME (EXPENSE): | ||||||||||||||||||||||
| Interest expense | (130,773 | ) | (727,137 | ) | (437,379 | ) | (429,964 | ) | (237,177 | ) | ||||||||||||
| Other, primarily interest income from related party in 1998 and 1999 | 88,303 | 2,393,368 | 2,062,887 | 2,056,480 | 144,312 | |||||||||||||||||
| Total interest and other income (expense) | (42,470 | ) | 1,666,231 | 1,625,508 | 1,626,516 | (92,865 | ) | |||||||||||||||
| INCOME (LOSS) BEFORE INCOME | ||||||||||||||||||||||
| TAXES | 1,529,282 | 5,070,327 | 5,091,848 | 3,064,478 | (5,261,303 | ) | ||||||||||||||||
| PROVISION FOR INCOME TAXES | 212,738 | — | — | — | — | |||||||||||||||||
| NET INCOME (LOSS) | $ | 1,316,544 | $ | 5,070,327 | $ | 5,091,848 | $ | 3,064,478 | $ | (5,261,303 | ) | |||||||||||
| PRO FORMA DATA (UNAUDITED), (NOTE 3): | ||||||||||||||||||||||
| Historical net income (loss) | — | — | $ | 5,091,848 | — | $ | (5,261,303 | ) | ||||||||||||||
| Pro forma provision for income taxes | — | — | 1,616,000 | — | 517,000 | |||||||||||||||||
| Pro forma net income (loss) | — | — | $ | 3,475,848 | — | $ | (5,778,303 | ) | ||||||||||||||
| Pro forma net income (loss) per common share (basic and diluted) | — | — | $ | 0.16 | — | $ | (0.26 | ) | ||||||||||||||
| Pro forma weighted-average common shares (basic and diluted) | — | — | 22,064,518 | — | 22,064,518 | |||||||||||||||||
See notes to consolidated financial statements.
F-4
Applied Epi, Inc. and Subsidiaries
| Note | |||||||||||||||||||||||||
| Receivable | Total | ||||||||||||||||||||||||
| Additional | Deferred | From | Stockholders’ | ||||||||||||||||||||||
| Common | Paid-in | Stock-Based | Retained | Related | Equity | ||||||||||||||||||||
| Stock | Capital | Compensation | Earnings | Party | (Deficit) | ||||||||||||||||||||
| Balance at January 1, 1997 | $ | 218,178 | $ | 1,010,732 | — | $ | 2,025,584 | — | $ | 3,254,494 | |||||||||||||||
| Net income | — | — | — | 1,316,544 | — | 1,316,544 | |||||||||||||||||||
| Advances to related parties | — | — | — | — | $ | (2,296,000 | ) | (2,296,000 | ) | ||||||||||||||||
| Stock sale | 15,052 | 334,948 | — | — | — | 350,000 | |||||||||||||||||||
| Purchase of stock options in connection with 1995 business acquisition | — | (69,900 | ) | — | — | — | (69,900 | ) | |||||||||||||||||
| Stock purchase and retirement | (17,200 | ) | (627,800 | ) | — | — | — | (645,000 | ) | ||||||||||||||||
| Balance at December 31, 1997 | 216,030 | 647,980 | — | 3,342,128 | (2,296,000 | ) | 1,910,138 | ||||||||||||||||||
| Net income | — | — | — | 5,070,327 | — | 5,070,327 | |||||||||||||||||||
| Advances to related parties (net of discount) | — | — | — | — | (3,973,168 | ) | (3,973,168 | ) | |||||||||||||||||
| Distributions to stockholders | — | — | — | (3,976,542 | ) | — | (3,976,542 | ) | |||||||||||||||||
| Distribution of Spectracom stock to the Company’s majority stockholder | — | — | — | (3,137,373 | ) | — | (3,137,373 | ) | |||||||||||||||||
| Balance at December 31, 1998 | 216,030 | 647,980 | — | 1,298,540 | (6,269,168 | ) | 4,106,618 | ||||||||||||||||||
| Net income | — | — | — | 5,091,848 | — | 5,091,848 | |||||||||||||||||||
| Advances to related parties | (2,117,038 | ) | (2,117,038 | ) | |||||||||||||||||||||
| Repayments from related parties (net of discount) | 8,386,206 | 8,386,206 | |||||||||||||||||||||||
| Balance at December 31, 1999 | 216,030 | 647,980 | — | 6,390,388 | — | 7,254,398 | |||||||||||||||||||
| Net loss (unaudited) | — | — | — | (5,261,303 | ) | — | (5,261,303 | ) | |||||||||||||||||
| Deferred stock-based compensation (unaudited) | — | 11,930,882 | $ | (11,930,882 | ) | — | — | — | |||||||||||||||||
| Amortization of deferred stock-based compensation (unaudited) | — | — | 1,729,414 | — | — | 1,729,414 | |||||||||||||||||||
| Majority stockholder sale deemed capital contribution (unaudited) | — | 5,494,699 | — | — | — | 5,494,699 | |||||||||||||||||||
| Distributions to stockholders (unaudited) | — | — | — | (217,883 | ) | — | (217,883 | ) | |||||||||||||||||
| Balance at June 30, 2000 (unaudited) | $ | 216,030 | $ | 18,073,561 | $ | (10,201,468 | ) | $ | 911,202 | $ | — | $ | 8,999,325 | ||||||||||||
See notes to consolidated financial statements.
F-5
Applied Epi, Inc. and Subsidiaries
| Six Months | |||||||||||||||||||||||
| Year Ended December 31, | Ended June 30, | ||||||||||||||||||||||
| 1997 | 1998 | 1999 | 1999 | 2000 | |||||||||||||||||||
| (unaudited) | |||||||||||||||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | |||||||||||||||||||||||
| Net income (loss) | $ | 1,316,544 | $ | 5,070,327 | $ | 5,091,848 | $ | 3,064,478 | $ | (5,261,303 | ) | ||||||||||||
| Adjustments to reconcile net income (loss) to net cash provided by operating activities: | |||||||||||||||||||||||
| Depreciation and amortization | 238,401 | 242,021 | 292,733 | 111,437 | 221,872 | ||||||||||||||||||
| Stock-based compensation | — | — | — | — | 7,224,113 | ||||||||||||||||||
| Discount on note receivable from related party | — | (1,605,373 | ) | (1,532,000 | ) | (1,532,000 | ) | — | |||||||||||||||
| Decrease in deferred taxes | 175,930 | — | — | — | — | ||||||||||||||||||
| Loss on sale of property and equipment | — | 43,483 | — | — | 80 | ||||||||||||||||||
| Changes in operating assets and liabilities: | |||||||||||||||||||||||
| Accounts receivable | (325,579 | ) | (1,445,007 | ) | (433,786 | ) | 949,397 | 722,211 | |||||||||||||||
| Inventories | (636,955 | ) | 265,386 | (843,939 | ) | (120,062 | ) | (3,181,397 | ) | ||||||||||||||
| Prepaid expenses and advances | 634,132 | (50,256 | ) | 129,352 | 83,748 | (29,730 | ) | ||||||||||||||||
| Accounts payable | (36,297 | ) | 28,049 | 174,542 | 253,728 | 696,721 | |||||||||||||||||
| Deferred revenue | 128,174 | (110,185 | ) | 81,677 | (756,660 | ) | 1,235,785 | ||||||||||||||||
| Accrued expenses | (144,817 | ) | 445,895 | 406,345 | (56,173 | ) | 318,574 | ||||||||||||||||
| Net cash provided by operating activities | 1,349,533 | 2,884,340 | 3,366,772 | 1,997,893 | 1,946,926 | ||||||||||||||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | |||||||||||||||||||||||
| Acquisition of property and equipment | (71,033 | ) | (121,461 | ) | (7,274,422 | ) | (21,154 | ) | (2,867,675 | ) | |||||||||||||
| Proceeds from sale of property and equipment | 11,805 | 4,142 | — | — | — | ||||||||||||||||||
| Payments from related party on note receivable | — | — | 9,918,206 | 9,918,206 | — | ||||||||||||||||||
| Advances to related party on note receivable | (2,296,000 | ) | (5,505,168 | ) | (2,117,038 | ) | (2,117,038 | ) | — | ||||||||||||||
| Net cash (used in) provided by investing activities | (2,355,228 | ) | (5,622,487 | ) | 526,746 | 7,780,014 | (2,867,675 | ) | |||||||||||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | |||||||||||||||||||||||
| Proceeds from issuance of notes payable | 2,000,000 | 6,200,000 | 5,100,000 | — | 2,335,686 | ||||||||||||||||||
| Proceeds from issuance of notes payable — related parties | — | 1,635,000 | 500,000 | — | — | ||||||||||||||||||
| Payments on notes payable | — | (386,057 | ) | (7,313,943 | ) | (7,313,943 | ) | (45,809 | ) | ||||||||||||||
| Payments on notes payable — related parties | — | — | (2,135,000 | ) | (2,100,000 | ) | (500,000 | ) | |||||||||||||||
| Payments on notes payable — former stockholders | (785,000 | ) | (610,042 | ) | (255,648 | ) | — | (244,210 | ) | ||||||||||||||
| Payments for loan origination fees | — | — | (109,378 | ) | — | — | |||||||||||||||||
| Proceeds from sale of stock | 350,000 | — | — | — | — | ||||||||||||||||||
| Distributions to stockholders | — | (3,976,542 | ) | — | — | (217,883 | ) | ||||||||||||||||
| Payments from (advances to) related party | 72,558 | (2,826 | ) | — | — | — | |||||||||||||||||
| Net cash provided by (used in) financing activities | 1,637,558 | 2,859,533 | (4,213,969 | ) | (9,413,943 | ) | 1,327,784 | ||||||||||||||||
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | 631,863 | 121,386 | (320,451 | ) | 363,964 | 407,035 | |||||||||||||||||
| CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD | 1,045,666 | 1,677,529 | 1,798,915 | 1,798,915 | 1,478,464 | ||||||||||||||||||
| CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 1,677,529 | $ | 1,798,915 | $ | 1,478,464 | $ | 2,162,879 | $ | 1,885,499 | |||||||||||||
| SUPPLEMENTAL CASH FLOW INFORMATION: | |||||||||||||||||||||||
| Interest paid | $ | 130,773 | $ | 739,641 | $ | 499,310 | $ | 491,746 | $ | 23,702 | |||||||||||||
| Income taxes refunded | 596,326 | — | — | — | — | ||||||||||||||||||
| Distribution of Spectracom stock to the Company’s majority stockholder | — | 3,137,373 | — | — | — | ||||||||||||||||||
| Notes payable issued to repurchase common stock | 714,900 | — | — | — | — | ||||||||||||||||||
See notes to consolidated financial statements.
F-6
Applied Epi, Inc. and Subsidiaries
| 1. | Nature of Business and Basis of Presentation |
Applied Epi, Inc. was incorporated in Minnesota in 1986 and was 93%, 93%, and 90% owned by the Majority Stockholder at December 31, 1997, 1998, and 1999, respectively. The Company is a provider of epitaxial equipment and related components used to produce compound semiconductors for the fiber optic and wireless markets, as well as other consumer applications.
In 1992, Applied Epi International, Inc. was incorporated as an Interest Charge Domestic International Sales Corporation (IC-DISC). Applied Epi International, Inc., was formed to perform foreign sales activities and to obtain tax benefits related to the Company’s foreign sales.
In 1993, Applied Epi Europe, Inc. was incorporated as a Minnesota company. Applied Epi Europe, Inc. was formed for the purpose of sales and distribution of the Company’s products in Europe.
On September 25, 2000, the Company entered into a tax-free transaction pursuant to Section 351 of the Internal Revenue Code and issued 97,604 shares of common stock to the Majority Stockholder in exchange for 100% of the shares of Applied Epi International, Inc. and Applied Epi Europe, Inc., resulting in these entities becoming wholly owned subsidiaries of the Company. Since Applied Epi International, Inc. and Applied Epi Europe, Inc. were under the common control of the Company due to the Majority Stockholder’s controlling interest in all three entities, the transaction has been accounted for on an “as if pooling” basis. The accompanying consolidated financial statements represent the financial position, results of operations, and cash flows of the Company, Applied Epi International, Inc. and Applied Epi Europe, Inc. for the period from January 1, 1997 to June 30, 2000 as if the transaction had occurred at the beginning of the earliest period presented. In addition, on September 19, 2000, the Board of Directors declared a four-for-one stock split effective immediately prior to the effectiveness of the Company’s planned initial public offering. All stock option, performance unit, share, per unit, and per share data have been restated to reflect the split. All significant intercompany accounts and activities have been eliminated in these consolidated financial statements.
| 2. | Summary of Significant Accounting Policies |
Unaudited Interim Financial Statements — The consolidated balance sheet as of June 30, 2000, the consolidated statements of operations and cash flows for the six-month periods ended June 30, 1999 and 2000, and the consolidated statement of stockholders’ equity for the six-month period ended June 30, 2000 have been prepared by the Company without audit. The amounts as of and for the six months ended June 30, 2000 included within the Notes to Consolidated Financial Statements have also been prepared by the Company without audit. In the opinion of the Company’s management, all adjustments (which include only normal recurring adjustments) necessary to present fairly the financial position, results of operations and cash flows, and changes in stockholders’ equity at June 30, 2000 and for the periods ended June 30, 1999 and June 30, 2000 have been made. Interim results are not necessarily indicative of the results that will be achieved for the year.
Management’s Use of Estimates — The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires
F-7
Business Risks — The nature of the Company’s operations exposes the Company to certain business risks. The markets for the Company’s products are competitive and subject to rapid technological change and evolving industry standards that may significantly alter the operation of the Company and its potential customers. Also, the Company is dependent on certain key employees.
Concentration of Credit Risk — Financial instruments that potentially subject the Company to concentrations of credit risk consist of cash and cash equivalents and accounts receivable. A majority of the Company’s cash is invested in one financial institution and a money market mutual fund.
The Company had two different customers that accounted for 50%, 42%, and 33% of combined trade accounts receivable at December 31, 1998, 1999, and June 30, 2000, respectively. The Company had no customers who individually accounted for more than 10% of consolidated sales in 1997, 1998, 1999, or the six months ended June 30, 2000. During 1997, 1998, 1999 and the six months ended June 30, 2000, the Company’s top ten customers accounted for an aggregate of 45%, 38%, 46%, and 52% of sales, respectively.
The Company periodically reviews all customer accounts receivable for collectibility. It generally does not require collateral for its trade accounts receivable. The Company manages credit risk by evaluating creditworthiness regularly. Estimated losses within accounts receivable are reserved for through establishment of an allowance for doubtful accounts. Customer accounts considered by management to be uncollectible are written off.
Revenue Recognition — The Company currently manufactures low volume production and research Molecular Beam Epitaxy (MBE) systems and performance products (effusions cells, software, and accessories). The Company’s revenue recognition policies are in accordance with the Securities and Exchange Commission’s (SEC) Staff Accounting Bulletin (SAB) No. 101, Revenue Recognition in Financial Statements. The Company’s revenue recognition policies for its products are as follows:
| Low Volume Production and Research Systems — For the majority of these systems, the customer is invoiced a portion of the contract price upon receipt of a signed purchase order (generally 20%). Once the Company manufactures the system, the customer witnesses a factory acceptance test at the Company’s facility and signs an acceptance form. The low volume production and research system is then disassembled, shipped to the customer’s site, and reassembled by company and customer personnel. Upon shipment, an additional portion of the contract price is billed to the customer (generally 70%). Once the system has been reassembled at the customer’s plant, generally a final site acceptance test is performed and the customer signs a final acceptance form. Upon receipt of final customer acceptance, the remaining unbilled contract price is invoiced to the customer (generally 10%) and all revenue from the contract is recognized. Until final customer acceptance has been received, amounts that have been billed to the customer are recorded as deferred revenue. At the time of final customer acceptance, a warranty reserve for the system is also established. | |
| Performance Products — Revenue is recognized upon shipment of product to the customer. Upon shipment, the customer is invoiced for the purchase price and payment is due within 30 days. There are no customer acceptance criteria associated with the sale of performance products. |
F-8
Cash and Cash Equivalents — The Company considers all highly liquid investments purchased with a maturity of three months or less to be cash equivalents.
Inventories — Inventories are stated at the lower of cost (first-in, first-out method) or market. The Company includes in inventory all direct material, labor, and production costs.
Property and Equipment — Property and equipment are recorded at historical cost and are being depreciated using both straight-line and accelerated methods over the following estimated useful lives:
| Building | 39 years | |||
| Equipment, furniture, and fixtures | 5-10 years |
Tax Increment Financing Receivable — On September 1, 1998, the Company entered into a development agreement with the Economic Development Authority of the Town of White Bear, Minnesota (the Authority) and Continental Technology Center, LLP (Continental). Under the terms of the agreement, the Authority was to pay Continental tax increment assistance on a pay-as-you-go basis commencing February 1, 2001. The Authority was to pay Continental 80% of the tax increments that it received and retained with respect to real estate taxes paid during the preceding calendar year. Such payments by the Authority were to continue until the earlier of (i) February 1, 2009 or (ii) until Continental had received the principal amount of $656,000, together with interest on the unpaid principal balance, at an annual rate of 9.75% (such interest to accrue from January 1, 1999).
The Company leased approximately 90% of the building built by Continental from July 1998 until it purchased the building and adjacent land from Continental for $6,825,000 in December 1999. In connection with this purchase, the tax increment financing arrangement was assigned to the Company. In connection with this assignment, the Company recorded a receivable in the amount of the expected future payments to be received of $719,960 and reduced its basis in the building by that amount.
Goodwill — Goodwill of $1,301,583 is related to the 1995 acquisition of Superior Vacuum Technology, Inc. (SVT) and is shown in the balance sheets net of accumulated amortization of $455,553, $585,712, and $650,791 at December 31, 1998, 1999, and June 30, 2000, respectively. Goodwill is being amortized on a straight-line basis over ten years. The Company periodically reviews goodwill to assess recoverability. The Company evaluates the recoverability by measuring the unamortized balance of such goodwill against estimated future cash flows. If events or circumstances indicated that the carrying amount of such asset may not be recoverable, goodwill would be adjusted to the present value of the estimated future cash flows. Based on evaluations performed, there was no adjustment to the carrying value of goodwill during 1997, 1998, 1999, or the six months ended June 30, 2000.
Impairment of Long-Lived Assets — The Company evaluates its long-lived assets (including certain property and equipment and goodwill) for impairment if events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. The Company evaluates the recoverability of long-lived assets by measuring the unamortized balance of the long-lived assets against estimated undiscounted future cash flow. If such analysis were to indicate that the carrying amount of such assets may not be recoverable, the assets would be adjusted to estimated fair value based on the present value of the estimated future cash flows. There was no adjustment to the carrying value of long-lived assets in 1997, 1998, 1999, or the six months ended June 30, 2000.
Accrued Warranty Expenses — The Company warrants its system and nonsystem products for periods of one and two years, respectively. The Company provides for estimated and known warranty costs through a warranty reserve. The warranty reserve of $148,996, $146,881, and $182,455 at
F-9
Research and Development — The Company incurs costs related to research and development in connection with new product development and the enhancement of existing products. Research and development costs consist primarily of wages, related employee benefits, and materials and are charged to expense as incurred.
Income Taxes — The Company is currently a Subchapter S Corporation under the Internal Revenue Code. As a result, any income tax liability is the sole responsibility of the individual stockholders, and no provision for income taxes (other than the provision for income taxes in 1997 which reflects the conversion to a Subchapter S Corporation beginning in that year) or income tax liability is recorded in the historical financial statements. If the Company completes a public offering of its common stock (the Offering), the Subchapter S election will be terminated, and the Company will account for income taxes in accordance with Statement of Financial Accounting Standards (SFAS) No. 109, Accounting for Income Taxes (see Note 3).
Stock-Based Compensation — The Company accounts for stock-based employee compensation arrangements in accordance with the provisions of Accounting Principles Board (APB) Opinion No. 25, Accounting for Stock Issued to Employees, and complies with the disclosure of SFAS No. 123, Accounting for Stock-Based Compensation. Accordingly, compensation expense has been recorded based on the intrinsic value amortized over the vesting period. In addition, the Company has adopted Financial Accounting Standards Board (FASB) Interpretation No. (FIN) 44, Accounting for Certain Transactions Involving Stock Compensation — an Interpretation of APB Opinion No. 25.
New Accounting Standards — SFAS No. 133, Accounting for Derivative Instruments and Hedging Activities, was issued in June 1998 and establishes accounting and reporting standards for derivative instruments. The statement requires recognition of all derivatives as either assets or liabilities in the balance sheet measured at fair value and will be effective in fiscal year 2001. The Company does not anticipate that the adoption of SFAS No. 133 will have a significant impact on the Company’s financial position or results of operations.
| 3. | Pro Forma Information (Unaudited) |
Pro Forma Balance Sheet — The pro forma balance sheet of the Company as of June 30, 2000 reflects the net deferred tax asset, recorded in accordance with SFAS No. 109, estimated to be $677,348, which will be recorded by the Company as a result of the termination of its S Corporation status in conjunction with the effective date of the Offering. The deferred tax asset will represent the tax effect of the cumulative differences between the financial reporting and income tax bases of certain assets and liabilities as of the termination date of S corporation status, and will be recorded as an income tax benefit in the quarter in which the S corporation status is terminated. The actual deferred tax asset recorded will be adjusted to reflect the effect of operations of the Company through the termination of its S corporation status. In addition, retained earnings have been recapitalized to additional paid-in capital upon the Company’s conversion to a C corporation.
F-10
The significant items comprising the Company’s pro forma net deferred tax asset as of June 30, 2000 are as follows:
| Current: | |||||
| Accrued vacation | $ | 60,000 | |||
| Employee performance plan, current | 224,000 | ||||
| Accrued warranty expenses | 70,000 | ||||
| Inventory | 85,000 | ||||
| 439,000 | |||||
| Noncurrent: | |||||
| Employee performance plan, noncurrent | 303,000 | ||||
| Goodwill | 247,000 | ||||
| Depreciation | (11,000 | ) | |||
| 539,000 | |||||
| Net deferred tax asset | $ | 978,000 | |||
Pro Forma Statement of Operations — If the Company completes the Offering, the Company will terminate its status as an S corporation and will be subject to federal and state income taxes thereafter. Accordingly, for informational purposes, the accompanying statements of income for the year ended December 31, 1999 and the six-months ended June 30, 2000 include an unaudited pro forma adjustment for the income taxes which would have been recorded if the Company had not been an S corporation, based on the tax laws in effect during the respective periods.
The differences between the federal statutory income tax rate and the pro forma income tax rate for the year ended December 31, 1999 and the six months ended June 30, 2000 are as follows:
| 1999 | 2000 | |||||||
| Federal statutory tax rate | 34 | % | (34 | )% | ||||
| State income taxes, net of federal benefit | 4 | (4 | ) | |||||
| Expenses not deductible for tax purposes (including $7,224,113 of nondeductible deferred stock-based compensation expense in 2000) | — | 55 | ||||||
| Income tax benefit of IC-DISC | (6 | ) | (6 | ) | ||||
| Other | — | (1 | ) | |||||
| 32 | % | 10 | % | |||||
Pro Forma Net Income (Loss) Per Share — Pro forma net income (loss) per share was calculated by dividing pro forma net income (loss) by the weighted average number of shares of common stock outstanding for the respective periods (as discussed in Note 1), and the pro forma shares which will be issued to pay the previously taxed but undistributed S corporation earnings. The Company estimates that the distribution of previously undistributed earnings retained prior to the conversion to a C corporation would have been approximately $6.0 million if the conversion had occurred on June 30, 2000. The actual amount will be determined after giving effect to the Company’s operating results through the S corporation termination date.
F-11
A reconciliation of pro forma shares used to compute basic and diluted income (loss) per share is as follows:
| December 31, | June 30, | |||||||
| 1999 | 2000 | |||||||
| (Unaudited) | ||||||||
| Weighted average number of shares of common stock outstanding | 21,602,980 | 21,602,980 | ||||||
| Pro forma shares for S corporation distributions | 461,538 | 461,538 | ||||||
| 22,064,518 | 22,064,518 | |||||||
| 4. | Segments |
For financial reporting purposes, management considers all of the Company’s operating activities as one reportable segment due to commonality in products, production process, types or classes of customers, and method of product distribution.
Sales by geographic area (allocated by the customer’s location) were as follows:
| Six Months | ||||||||||||||||
| Year Ended December 31, | Ended | |||||||||||||||
| June 30, | ||||||||||||||||
| 1997 | 1998 | 1999 | 2000 | |||||||||||||
| (Unaudited) | ||||||||||||||||
| United States | $ | 8,001,519 | $ | 10,116,389 | $ | 9,281,145 | $ | 6,331,793 | ||||||||
| Other countries | 3,852,053 | 5,416,548 | 6,107,796 | 3,847,977 | ||||||||||||
| $ | 11,853,572 | $ | 15,532,937 | $ | 15,388,941 | $ | 10,179,770 | |||||||||
| 5. | Inventories |
Inventories consist of the following:
| December 31, | ||||||||||||
| June 30, | ||||||||||||
| 1998 | 1999 | 2000 | ||||||||||
| (Unaudited) | ||||||||||||
| Raw materials | $ | 1,384,634 | $ | 1,379,734 | $ | 2,879,882 | ||||||
| Work-in-progress | 408,020 | 1,030,018 | 2,924,365 | |||||||||
| Finished goods including systems awaiting final customer acceptance of $304,145, $475,549, and $340,000 in 1998, 1999, and 2000, respectively | 344,234 | 571,075 | 357,977 | |||||||||
| $ | 2,136,888 | $ | 2,980,827 | $ | 6,162,224 | |||||||
F-12
| 6. | Debt |
Long-term debt consists of the following at December 31:
| December 31, | ||||||||||||
| June 30, | ||||||||||||
| 1998 | 1999 | 2000 | ||||||||||
| (Unaudited) | ||||||||||||
| Term note payable, due in monthly installments through December 2019, variable interest rate (8.51% and 8.69% at December 31, 1999 and June 30, 2000, respectively) | — | $ | 4,500,000 | $ | 4,454,190 | |||||||
| Term note payable, due December 2001, variable interest rate (8.5% and 9.5% at December 31, 1999 and June 30, 2000, respectively) | — | 600,000 | 600,000 | |||||||||
| Revolving note payable to bank, due July 2001, variable interest rate (8.57% at June 30, 2000) | — | — | 2,200,687 | |||||||||
| Note payable to economic development authority, due December 2008, 7.5% interest rate at June 30, 2000 | — | — | 135,000 | |||||||||
| Notes payable to former stockholders, paid January 2000 | $ | 499,858 | 244,210 | — | ||||||||
| Revolving note payable to bank, paid June 1999 | 1,600,000 | — | — | |||||||||
| Term note payable to bank, paid June 1999 | 5,713,943 | — | — | |||||||||
| 7,813,801 | 5,344,210 | 7,389,877 | ||||||||||
| Less current portion | 7,813,801 | 325,914 | 2,282,391 | |||||||||
| $ | — | $ | 5,018,296 | $ | 5,107,486 | |||||||
Long-term debt maturities at December 31, 1999 were as follows:
| 2000 | $ | 325,914 | ||
| 2001 | 697,368 | |||
| 2002 | 105,985 | |||
| 2003 | 115,365 | |||
| 2004 | 125,575 | |||
| 2005 and thereafter | 3,974,003 | |||
| $ | 5,344,210 | |||
Revolving Credit Agreement — In June 2000, the Company entered into a revolving credit agreement with U.S. Bank, National Association (the Revolver). The Revolver consists of a revolving loan of up to $2,500,000. The Revolver requires the Company to pay interest on the outstanding debt on a monthly basis at a floating rate equal to the sum of (a) the adjusted Eurodollar rate (as defined) plus the applicable margin (as defined). The Company’s borrowing base under the Revolver is equal to the sum of (1) the lesser of (x) 50% of eligible inventory or (y) $1,500,000 plus (2) 80% of eligible receivables. The outstanding principal together with all unpaid interest is due on July 1, 2001. As of June 30, 2000, the outstanding balance under the Revolver was $2,200,687 and the amount available for additional borrowings was $299,313. The Revolver is guaranteed by the Majority Stockholder.
The Revolver is secured by virtually all of the Company’s inventory, equipment, and general intangibles. Also, the Revolver requires the Company to meet certain affirmative and negative covenants. As of June 30, 2000, the Company was in compliance with the financial covenants contained in the Revolver.
F-13
Credit Agreements — In December 1999, the Company entered into a credit agreement with Jackson National Life Insurance Company. The credit agreement consists of a $4,500,000 note which is payable over a term of 20 years. The note requires monthly payments of principal and interest of $39,081 at the greater of the annual LIBOR rate plus 2.05% or 6%. The proceeds of the note were used for the purchase of land and the Company’s building in Saint Paul, Minnesota. The note is secured by a lien on the land and building and a security interest in certain property, fixtures, and equipment. The note is also guaranteed by the Majority Stockholder.
In December 1999, the Company entered into a credit agreement with North American Banking Company (NABC). The agreement consists of a $600,000 note which is payable over a term of two years. The note requires monthly interest payments at NABC’s prime rate. The proceeds of the note were used for the purchase of land. The credit agreement requires the Company to maintain certain financial covenants as to minimum tangible net worth and current ratio. As of June 30, 2000, the Company was in compliance with the financial covenants contained in the credit agreement.
Notes Payable — Former Stockholders — On March 18, 1997, in a noncash transaction, the Company repurchased 1,720,000 shares of its common stock and 932,000 common stock options that were issued in 1995 as consideration for the acquisition of SVT. In connection with a put option contained in the purchase agreement with SVT, the Company paid $0.375 per share and $0.075 per option, for a total of $714,900. For payment of the shares and options, the Company signed various promissory notes providing for quarterly interest payments until the notes are paid in full. Interest is charged at 8.5% per annum. The amount outstanding on these notes as of December 31, 1998 and 1999 was $499,858 and $244,210, respectively. The notes were fully repaid in January 2000.
Notes Payable — Related Parties — In 1998, the Majority Stockholder advanced the Company $1.6 million. The note payable required monthly payments of interest only at prime plus 0.5%. This note was repaid in June 1999.
In 1998, an officer of the Company advanced the Company $35,000. This note was repaid in June 1999.
In 1999, the Majority Stockholder advanced the Company $500,000. The note payable requires monthly payments of interest only at prime plus 0.5% (9% as of December 31, 1999) until maturity on December 16, 2000. This note was repaid in April 2000.
7. Related-Party Transactions and Agreements
Due from Related Party — During 1998, the Company advanced an additional $2,826 to NuVision LLC. As of December 31, 1999 and 1998, the outstanding balance due from NuVision LLC was $589,932. NuVision LLC and the Company have the same Majority Stockholder. The balance due was received in April 2000.
Notes Receivable and Stock Received from Related Party — During 1998 and until June 1999, the Company utilized its credit facility to advance funds to Spectracom, Inc. (Spectracom) which was majority owned by the Company’s majority stockholder. In June 1999, Spectracom was sold to an unrelated third party and all amounts due to the Company were repaid. The advances to Spectracom were at a favorable interest rate to Spectracom, for which Spectracom compensated the Company by issuing 3,070,880 shares of Spectracom’s common stock to the Company. Management of the Company estimated the fair value of the stock granted to the Company was approximately $3,137,000, of which $1,605,000 was recorded as interest income in 1998 with the remaining amount of $1,532,000 reflected in the December 31, 1998 balance sheet as discount on notes receivable from related party. Upon receipt of the Spectracom stock, the Company distributed the Spectracom stock to the Company’s stockholders. This discount was recorded as interest income ratably by the
F-14
Equipment Purchases by Related Party — During 1998, the Company recognized $1.8 million in revenue from the sale of MBE products to Spectracom for use in research and development and product manufacturing operations. The prices charged by the Company to Spectracom were substantially the same as those charged to unrelated customers of the Company.
Cost Reimbursement — During 1998, the Company was reimbursed by Spectracom for certain costs incurred on behalf of Spectracom in the amount of approximately $179,000. Such reimbursement has been netted against selling expenses in the 1998 statement of operations.
Majority Stockholder Sales of Common Stock — In April 2000, the majority shareholder sold 569,112 common shares of the Company to four current employees at $1.40 per share. This transaction has been accounted for in accordance with the AICPA’s Accounting Interpretation No. 1 of APB Opinion No. 25. Accordingly, a charge to compensation expense was made to the extent the price was below the fair value of the stock shares sold and a corresponding entry was made to record additional paid-in capital of $5,494,699 in the six-month period ended June 30, 2000.
8. Stock Option Plan
1993 Stock Option Plan — In June 1993, the Board of Directors approved a Stock Option Plan under which the Company has reserved 6,000,000 shares of common stock for the issuance of incentive stock options (ISO) and nonqualified stock options (NQO) to officers and other key employees. The provisions of the plan, as amended, state that the term of any ISO shall be fixed by the Board. However, no ISO shall be exercisable more than ten years after the date of grant. The term of any ISO granted to an employee who owns more than 10% of the combined voting power of all classes of stock of the Company is limited to no more than five years from the date of grant.
For employees who do not own more than 10% of the combined voting power of all classes of stock of the Company, the ISO exercise price shall not be less than fair value at the date of grant. For employees who own more than 10% of the combined voting power of all classes of stock of the Company, the ISO exercise price shall not be less than 110% of the fair value at the date of grant. The NQO exercise price per share is any price deemed appropriate by the Board.
There were no options outstanding at December 31, 1999.
As of December 31, 1999 and June 30, 2000, there were 6,000,000 and 4,413,752 shares available for grant under the 1993 Stock Option Plan, respectively.
The following summarizes information for options granted and outstanding at June 30, 2000:
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| Weighted | Weighted | Weighted | ||||||||||||||||||
| Average | Average | Average | ||||||||||||||||||
| Number of | Remaining | Exercise | Number of | Exercise | ||||||||||||||||
| Range of Prices | Shares | Life | Price | Shares | Price | |||||||||||||||
| $ 1.40 | 1,045,848 | 10 | $ | 1.40 | — | — | ||||||||||||||
| 7.50 | 516,400 | 10 | 7.50 | — | — | |||||||||||||||
| 12.50 | 24,000 | 10 | 12.50 | — | — | |||||||||||||||
| 1,586,248 | 10 | $ | 3.55 | — | — | |||||||||||||||
F-15
Stock-Based Compensation — Grants made during 2000 contained exercise prices which were management’s best estimate of fair value of the underlying common stock on the date of grant. However, subsequent to the grant date, management concluded that for certain grants which occurred after the Company’s development of a new high-volume MBE system may not have fully reflected the impact of this event. Management has concluded that for the grants made in April and May, the fair value per share on the date of grant was $11.05. For subsequent grants, management has concluded that the fair value per share was $12.50. During the six months ended June 30, 2000 compensation cost aggregated $11,930,882, which will be amortized to expense over the three-year vesting period of the options. This compensation is being recognized on an accelerated basis using the model presented in paragraph 24 of FASB Interpretation No. (FIN) 28. Accordingly, amortization of these options which vest over three years will be approximately 48%, 37%, and 15%, respectively. For the six months ended June 30, 2000, compensation expense related to these grants aggregated $1,729,414.
As described in Note 2, the Company uses the intrinsic value method to measure compensation expense associated with grants of stock options to employees. If the Company had used the fair value method to measure compensation, the Company’s pro forma net loss and loss per share would have been $5,881,118 and $(0.26), respectively, for the six months ended June 30, 2000.
The fair value of each option grant was estimated on the grant date using the Black-Scholes option pricing model with the following weighted average assumptions during the six months ended June 30, 2000:
| Expected life of option grants | 3 years | |||
| Risk-free interest rate | 6.0% | |||
| Volatility | 0.0% | |||
| Dividend yield | 0.0% |
The Black-Scholes option pricing model was designed to value readily tradable stock options with relatively short lives. However, management believes that the assumptions used to value the options and the model applied yield a reasonable estimate of the fair value of the grants made under the circumstances.
9. Employee Performance Plan
The Company has a nonqualified employee performance plan. The plan provides for an annual discretionary award of performance units which is determined by the Board of Directors. The Board of Directors may terminate the plan at any time, and the plan shall automatically terminate in 2003. The performance units granted to a participant vest upon grant and are payable on the earlier of March 15 of the third calendar year after the year of grant; or a change in control of the Company due to sale, merger, or issuance of shares. The value per unit is determined at the end of each reporting period based on cumulative adjusted pretax profits, for the prior three years. Such payment is mandatory for former employees but may be deferred by current employees at their election. The Company recognizes expense for units granted in the current year and the change in the value of the unpaid units granted in prior years. Units exercised in a given year are paid in cash at the value per unit determined on the last calendar year-end. The Company expensed approximately $94,000, $376,500, $468,500, and $150,000 related to the plan during 1997, 1998, and 1999, and the six months ended June 30, 2000, respectively. During 1997, 1998, and 1999, and the six months ended June 30, 2000, the Company paid $38,892, $50,906, $104,305, and $22,648 to participants who redeemed 68,532, 90,500, 138,152, and 22,648 units, respectively. The value of a performance unit as of December 31, 1999 was approximately $1.00.
F-16
The following is a schedule of performance units outstanding under the plan as of December 31, 1999:
| Year Issued | Number of Units | |||
| 1992 | 51,388 | |||
| 1993 | 58,880 | |||
| 1994 | 167,496 | |||
| 1995 | 234,580 | |||
| 1996 | 106,908 | |||
| 1997 | 175,828 | |||
| 1998 | 260,648 | |||
| 1999 | 209,884 | |||
| 1,265,612 | ||||
As of December 31, 1999, there were 384,952 additional units available to be granted based on 2000 and future performance.
Upon the effectiveness of an initial public offering, the Company may, at its election, pay performance units by issuing its common stock, paying cash or any combination of common stock and cash. Accordingly, in the quarter in which the Company completes an initial public offering, the Company will incur compensation expense for the cash paid and fair value of a converted restricted common share less the amount previously expensed per performance unit times the number of restricted shares issued. The shares are restricted as to transferability. Management estimates that the compensation charge will be significant. The plan will be terminated upon the effectiveness of an initial public offering and no more units will be granted.
10. Commitments and Contingencies
In 1998, the Company entered into an operating lease for its facility. In December 1999, the Company purchased its facility and an adjacent lot for $6,825,000 and canceled the operating lease. Rent expense prior to the lease cancellation totaled approximately $171,000, $432,000, and $582,000 for the years ended December 31, 1997, 1998 and 1999, respectively.
In the ordinary course of business, the Company is subject to various lawsuits, claims, and proceedings relating to the conduct of its business. The Company records liabilities when loss amounts are determined to be probable and reasonably estimable. Although the outcome of litigation cannot be predicted with certainty and some lawsuits, claims, or proceedings may be disposed of unfavorably to the Company, management believes, based on facts presently known, that the outcome of such legal proceedings and claims will not have a material adverse effect on the Company’s financial position, liquidity, or future results of operations.
11. Income Taxes
Beginning with tax year 1997, Applied Epi, Inc. and Applied Epi Europe, Inc. have elected to be recognized as Subchapter S corporations under the provisions of the Internal Revenue Code and, as such, do not pay federal or state income taxes. The income from these corporations is included in the stockholders’ individual income tax returns, who are then responsible for the related income taxes.
The provision for income taxes for the year ended December 31, 1997 of $212,738 primarily consists of the effects of conversion to Subchapter S status, including the elimination of the deferred tax asset.
F-17
As an IC-DISC, Applied Epi International, Inc. is not required to pay federal corporate income taxes. Approximately $391,000, $565,000 and $838,000 of income from Applied Epi International, Inc. was not subject to income taxes in 1997, 1998 and 1999, respectively. As required to retain its status as an IC-DISC, it is the intention of management to reinvest such earnings indefinitely.
12. 401(k) Plan
The Company adopted a 401(k) plan effective April 1995. Employees become eligible to participate in the 401(k) plan after their first month of employment. The 401(k) plan is intended to qualify under Section 401(k) of the Internal Revenue Code (the Code), so that contributions to the 401(k) plan by employees or the Company and the investment earnings thereon are not taxable to the employees until withdrawn. If the 401(k) plan qualifies under Section 401(k) of the Code, the Company’s contributions will be deductible when made. Employees may elect to reduce their current compensation by up to the statutorily prescribed annual limit of $10,500 in 2000 and to have those funds contributed to the 401(k) plan. The 401(k) plan permits, but does not require, the Company to make additional matching contributions on behalf of all participants. During 1997, 1998, 1999, and the six months ended June 30, 2000, the Company did not make any contributions to the 401(k) plan.
13. Subsequent Events
Land Purchase — In June 2000, the Company purchased land adjacent to its present corporate headquarters in Saint Paul, Minnesota for $2,283,400. The Company intends to use the land for the construction and development of a process integration center.
Term Loan — In July 2000, the Company entered into a term loan credit agreement with U.S. Bank, National Association (the Term Loan). The Term Loan consists of a $1,650,000 note which is payable beginning September 1, 2000. The Term Loan requires monthly payments of principal and interest of $12,694. Interest on the outstanding debt is equal to a floating rate equal to the sum of the adjusted Eurodollar rate (as defined) plus 1.9%. The outstanding principal together with all unpaid interest is due on August 1, 2005.
The Term Loan is secured by a mortgage on the land purchased by the Company in June 2000 and all machinery, equipment, and personal property to be located on the land in the future. The Term Loan is also guaranteed by the Majority Stockholder.
2000 Stock Plan — In July 2000, the Board of Directors approved a Stock Plan under which the Company has reserved 6,000,000 shares of common stock for the issuance of incentive stock options (ISO), nonqualified stock options (NQO) and restricted stock to executives, key employees, consultants, and directors. The provisions of the plan state that the term of any ISO shall be fixed by the Board of Directors. However, no ISO shall be exercisable more than ten years after the date of grant. The term of any ISO granted to an individual who owns more than 10% of the combined voting power of all classes of stock of the Company is limited to no more than five years from the date of grant.
For individuals who do not own more than 10% of the combined voting power of all classes of stock of the Company, the ISO exercise price shall not be less than fair value at the date of grant. For individuals who own more than 10% of the combined voting power of all classes of stock of the Company, the ISO exercise price shall not be less than 110% of the fair value at the date of grant. The NQO exercise price per share is any price deemed appropriate by the Board of Directors.
F-18
Stock Options — In July 2000, the Company issued options to purchase 338,000 shares of common stock to an officer and certain key employees pursuant to the 1993 Stock Option Plan. The options have exercise prices of $10.00 and $12.50 per share, have a term of ten years, and vest over three years (one-third of the shares on each of the first three anniversaries of the date of grant).
In July and September 2000, the Company issued options to purchase 240,000 shares of common stock to two non-employee directors pursuant to the 2000 Stock Plan. The options have an exercise price of $12.50 per share; have a term expiring 30 days after the director’s retirement, resignation, or termination from the Board; and vest over three years (one-third of the shares on each of the first three anniversaries of the date of grant).
In August 2000, vesting was accelerated on 899,448 options originally issued in April 2000 to two officers of the Company. This accelerated vesting will result in a new measurement date and a non-cash compensation charge in the quarter ended September 30, 2000 of approximately $7,400,000.
F-19
Back Inside Cover Page:
The back inside cover page includes the heading “Global Services” and a picture of the globe depicting the locations of our sales offices and sales representatives. Below this picture is the following text:
“Applied Epi is a global company with state-of-the-art R&D and manufacturing facilities, plus an extensive service and support infrastructure. The company has direct sales and support offices in the U.S. and Europe. Sales representatives are located in China, France, India, Japan, Singapore and Taiwan.”
In the bottom right hand corner of the page is the Applied Epi logo.
Through and including , 2000 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Shares

Common Stock
Joint Book-Running Managers
| Donaldson, Lufkin & Jenrette | Merrill Lynch & Co. |
Wit SoundView
, 2000
Part II
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expense other than underwriting discounts and commissions, payable by Applied Epi, Inc. in connection with the sale of Common Stock being registered. All amounts are estimates except the SEC registration fee and the NASD filing fee.
| SEC registration fee | $ | 25,633 | ||
| NASD filing fee | $ | 10,210 | ||
| Nasdaq National Market listing fee | * | |||
| Printing and engraving costs | * | |||
| Legal fees and expenses | * | |||
| Accounting fees and expenses | * | |||
| Transfer Agent and Registrar fees | * | |||
| Miscellaneous expenses | * | |||
| Total | * |
| * | To be filed by amendment. |
Item 14. Indemnification of Directors and Officers
Section 302A.521 of the Minnesota Business Corporation Act (“MBCA”) provides that, unless prohibited or limited by a corporation’s articles of incorporation or bylaws, a corporation must indemnify its current and former officers, directors, employees and agents against reasonable expenses (including attorneys’ fees), judgments, penalties, fines and amounts paid in settlement and which were incurred in connection with actions, suits, or proceedings in which such persons are parties by reason of the fact that they are or were an officer, director, employee or agent of the corporation, if they: (1) have not been indemnified by another organization, (2) acted in good faith, (3) received no improper personal benefit, (4) in the case of a criminal proceeding, had no reasonable cause to believe the conduct was unlawful, and (5) reasonably believed that the conduct was in the best interests of the corporation. Section 302A.521 also permits a corporation to purchase and maintain insurance on behalf of its officers, directors, employees and agents of the corporation, whether or not the corporation would have been required to indemnify the person against the liability under the provisions of such section.
Article V of our restated and amended bylaws provides that we may indemnify each person who is or was a director, officer or employee to the full extent permitted by the MBCA. The purchase agreement (Exhibit 1.1) provides for indemnification by the underwriters of us, our directors and executive officers and other persons for certain liabilities, including liabilities arising under the Securities Act.
We maintain a policy of directors’ and officers’ liability insurance that insures our directors and officers against the cost of defense, settlement or payment of a judgment under certain circumstances. In conjunction with the effectiveness of the registration statement, we plan to expand its coverage to include securities law claims.
II-1
Item 15. Recent Sales of Unregistered Securities
Since September 25, 1997, we have issued unregistered securities to a limited number of persons as described below:
As of September 25, 2000, we had granted options to purchase an aggregate of 1,952,248 common shares to our employees pursuant to our 1993 Stock Option Plan, with exercise prices ranging from $1.395-$12.50 and options to purchase 242,000 shares of common stock to our non-employee directors pursuant to our 2000 Stock Plan at an exercise price of $12.50. We have not issued any shares pursuant to stock option exercises.
On September 25, 2000 we issued 97,604 shares of our common stock to Mr. Colombo in exchange for his 250 shares of Applied Epi International, Inc. and his 50,000 shares of Applied Epi Europe, Inc.
The sales of the above securities were deemed to be exempt from registration in reliance on Rule 701 promulgated under Section 3(b) under the Securities Act as transactions pursuant to a compensatory benefit plan or a written contract relating to compensation, or in reliance on Section 4(2) of the Securities Act promulgated thereunder as transactions by an issuer not involving any public offering. The recipients of securities in each such transaction represented their intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the share certificates and other instruments issued in such transactions. All recipients either received adequate information about Applied Epi or had access, through employment or other relationships, to such information. None of the transactions set forth in Item 15 involved underwritten offerings.
Item 16. Exhibits and Financial Statement Schedules
(a) Exhibits
| Exhibit | ||||
| Number | Description of Document | |||
| 1.1 | Form of Purchase Agreement. | |||
| 3.1 | * | Amended and Restated Articles of Incorporation. | ||
| 3.2 | * | Bylaws. | ||
| 4.1 | * | Form of stock certificate. | ||
| 5.1 | * | Opinion of Robins, Kaplan, Miller & Ciresi L.L.P. | ||
| 10.1 | 1993 Stock Option Plan. | |||
| 10.2 | 2000 Stock Plan. | |||
| 10.3 | Loan Agreement by and between Jackson National Life Insurance Company and Chorus Corporation, dated as of December 15, 1999. | |||
| 10.4 | $4,500,000 Promissory Note, dated December 15, 1999. | |||
| 10.5 | Credit Agreement by and between Chorus Corporation, Chorus International Corporation, Epi Europe, Ltd. and U.S. Bank National Association, dated as of June 5, 2000. | |||
| 10.6 | $2,500,000 Revolving Note to U.S. Bank National Association dated June 5, 2000. | |||
| 10.7 | Loan Agreement by and between Applied Epi, Inc. and U.S. Bank National Association dated July 31, 2000. | |||
II-2
| Exhibit | ||||
| Number | Description of Document | |||
| 10.8 | $1,650,000 Note by Applied Epi, Inc. in favor of U.S. Bank National Association dated July 31, 2000. | |||
| 10.9 | $600,000 Promissory Note by Chorus Corporation to North American Banking Company dated December 14, 1999. | |||
| 15.1 | * | Letter regarding unaudited interim financial information. | ||
| 21.1 | Subsidiaries of the registrant. | |||
| 23.1 | Consent of Deloitte & Touche LLP. | |||
| 23.2 | * | Consent of Counsel (see Exhibit 5.1). | ||
| 24.1 | Power of Attorney (see signature page). | |||
| 27.1 | Financial Data Schedules. | |||
* To be filed by amendment
(b) Financial Statement Schedules
See Exhibit 27.1
Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
Item 17. Undertakings
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the purchase agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions referenced in Item 14 of this registration statement or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by a director, officer or controlling person in connection with the securities being registered hereunder, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
II-3
| For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-4
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of St. Paul, MN, on the 28th day of September, 2000.
| APPLIED EPI, INC. | |
| /s/ David G. Reamer | |
|
|
|
| David G. Reamer, | |
| President and Chief Executive Officer |
Power of Attorney
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Paul E. Colombo and David G. Reamer and each of them, his attorneys-in-fact, each with the power of substitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this Registration Statement, and to sign any registration statement for the same offering covered by this Registration Statement that is to be effective upon filing pursuant to Rule 462(b) promulgated under the Securities Act of 1933, as amended, and all post-effective amendments thereto, and to file the same, with all exhibits thereto and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that such attorneys-in-fact and agents or any of them, or his or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement and Power of Attorney has been signed by the following persons in the capacities and as of the 28th day of September, 2000:
| Signature | Title | |
|
/s/ Paul E. Colombo Paul E. Colombo |
Chairman | |
|
/s/ David G. Reamer David G. Reamer |
President, Chief Executive Officer and Director (Principal Executive Officer) | |
|
/s/ Brett D. Heffes Brett D. Heffes |
Chief Financial Officer and Treasurer (Principal Financial and Accounting Officer) | |
|
/s/ Frank C. Kraemer Frank C. Kraemer |
Chief Operating Officer, Secretary and Director | |
|
/s/ Noel P. Rahn Noel P. Rahn |
Director | |
|
/s/ William J. Cadogan William J. Cadogan |
Director |
II-5
| Exhibit | ||||
| Number | Description of Document | |||
| 1.1 | Form of Purchase Agreement. | |||
| 3.1 | * | Amended and Restated Articles of Incorporation. | ||
| 3.2 | * | Bylaws. | ||
| 4.1 | * | Form of stock certificate. | ||
| 5.1 | * | Opinion of Robins, Kaplan, Miller & Ciresi L.L.P. | ||
| 10.1 | 1993 Stock Option Plan. | |||
| 10.2 | 2000 Stock Plan. | |||
| 10.3 | Loan Agreement by and between Jackson National Life Insurance Company and Chorus Corporation, dated as of December 15, 1999. | |||
| 10.4 | $4,500,000 Promissory Note, dated December 15, 1999. | |||
| 10.5 | Credit Agreement by and between Chorus Corporation, Chorus International Corporation, Epi Europe, Ltd. and U.S. Bank National Association, dated as of June 5, 2000. | |||
| 10.6 | $2,500,000 Revolving Note to U.S. Bank National Association dated June 5, 2000. | |||
| 10.7 | Loan Agreement by and between Applied Epi, Inc. and U.S. Bank National Association dated July 31, 2000. | |||
| 10.8 | $1,650,000 Note by Applied Epi, Inc. in favor of U.S. Bank National Association dated July 31, 2000. | |||
| 10.9 | $600,000 Promissory Note by Chorus Corporation to North American Banking Company dated December 14, 1999. | |||
| 15.1 | * | Letter regarding unaudited interim financial information. | ||
| 21.1 | Subsidiaries of the registrant. | |||
| 23.1 | Consent of Deloitte & Touche LLP. | |||
| 23.2 | * | Consent of Counsel (see Exhibit 5.1). | ||
| 24.1 | Power of Attorney (see signature page). | |||
| 27.1 | Financial Data Schedules. | |||
* To be filed by amendment
|
|